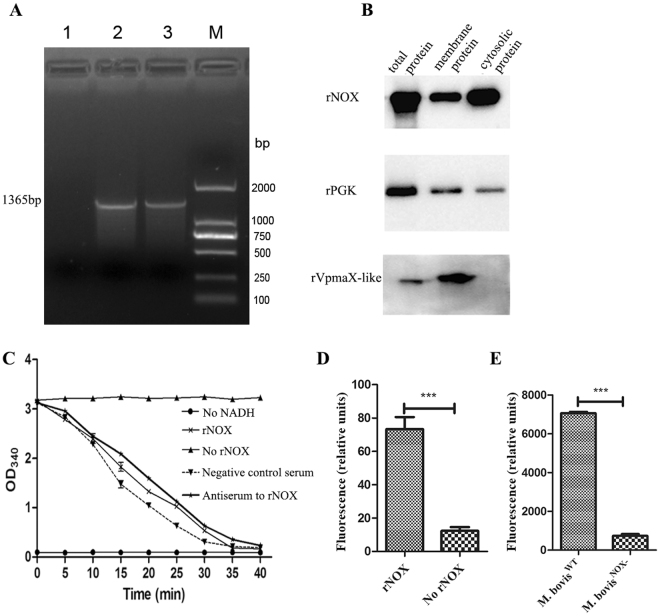
Figure 2.
The cloning, expression, and enzymatic activity of NADH oxidase (NOX). (A) PCR amplification of M. bovis nox gene. Lane 1: negative control; Lane 2 and Lane 3: The mutated nox gene of M. bovis. M: DNA marker. (B) The localization of NOX and PGK in M. bovis. The total proteins, membrane proteins and cytosolic proteins were incubated with mAbs to rNOX (1:1000), mAbs to rVpmaX-like protein (1:1000), and antiserum against PGK (1:500). The membrane protein rVmapX-like served as positive control. The cropped blots are displayed here, the full-length blots are presented in supplementary information (Fig. S4). (C) Enzymatic activity of purified rNOX at 5 μg/ml. The OD340 nm values of rNOX group decreased with the time increasing, whereas no reduction in absorbance was observed in negative control (No rNOX) and blank control (No NADH). 5 μl mouse antiserum to rNOX (1:200) incubated with rNOX before testing enzyme activity, while the equal amount of mixed negative serum served as negative control serum. Compared the antiserum to rNOX with negative control serum and rNOX, we found anti-rNOX polyclonal antibody cannot affect rNOX enzyme activity. (D) Enzymatic activity of rNOX. After rNOX converted NADH to NAD+, a kit was used to confirm whether rNOX produced H2O2. Compared to No rNOX, it was determined that rNOX could produce H2O2. (E) H2O2 production by M. bovis NOX− and M. bovis WT grown in glycerol as the carbon source. Statistical significance was determined by student t test (p < 0.001(***)).