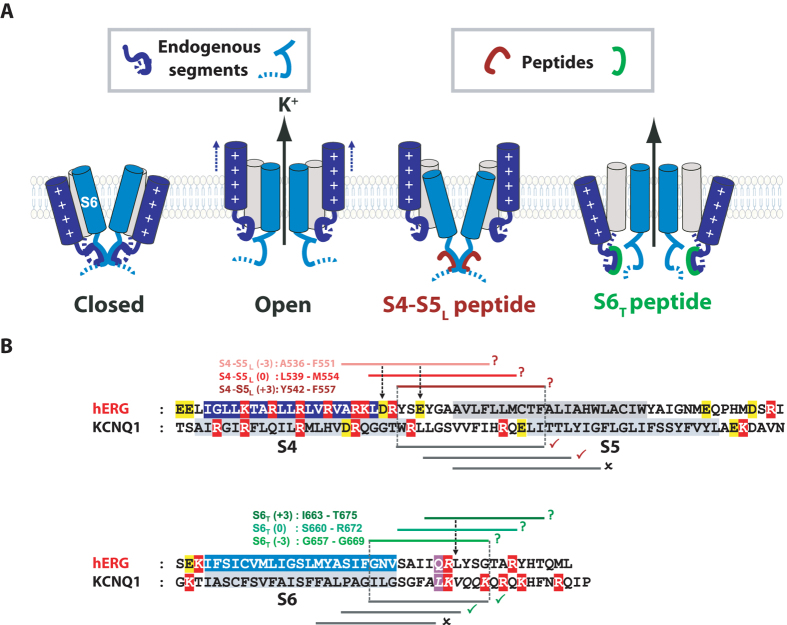
Figure 1.
Hypothetical ligand/receptor model. Alignment used to design S4-S5L and S6T peptides. (A) Scheme of the hypothetical ligand/receptor model in which S4-S5L (deep blue) binds to S6T (light blue) to stabilize the channel in a closed state. Upon membrane depolarization, S4 pulls S4-S5L out of the S6T receptor, allowing channel opening. The S4-S5L peptide (red) mimics endogenous S4-S5L, locking the channel in its closed conformation. Contrarily, S6T peptide (green) binds to the endogenous S4-S5L and limits its locking effect, leading to channel up-modulation. (B) Alignment used to design hERG peptides from previously identified KCNQ1 S4-S5L and S6T peptides (based on the multiple alignment obtained using Clustal Omega, presented in Supplemental Fig. 4). In red are represented the basic residues, in yellow acidic residues, and in purple the position of the narrowest part of the bundle crossing, also named the gating residue (see methods). The color boxes represent the transmembrane segments. Grey lines represent the peptides tested in KCNQ1 while red lines and green lines represent the designed hERG S4-S5L and S6T peptides, respectively. A check sign (✓) indicates that the KCNQ1 S4-S5L peptide inhibits the channel (red) and that the KCNQ1 S6T peptide activates the channel (green).