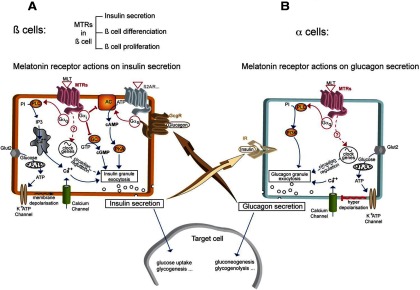
Figure 3.
Melatonin Receptors in Pancreatic α- and β-Cells. A, β-cells: In β-cells, the main signal for insulin secretion is the extracellular glucose concentration. Transporters (Glut2) transfer glucose to mitochondria, allowing ATP production. ATP causes the closure of K+ATP channels leading to membrane depolarization and activation of voltage-dependent L-type Ca++ channels. The subsequent intracellular calcium increase triggers insulin granule exocytosis and insulin liberation to the extracellular medium. Activated GPCRs coupled to Gαs, such as ß2-adrenergic receptor (β2AR) but also glucagon receptor (GcgR), lead to intracellular cAMP increase and activation of cAMP-dependent protein kinase (protein kinase A [PKA]) which is a secondary signal for insulin liberation. Melatonin receptors (MTRs) can interact with insulin secretion by activating different pathways leading to opposite effects: the predominant signal is given by activated MTRs coupled to Gαi which counteract cAMP production by inhibiting adenylyl cyclase (AC) or cGMP production by inhibiting guanylate cyclase (GC), thus leading to decrease of insulin secretion. In addition, MTRs can couple to Gαq to activate phospholipase C (PLC), increasing [Ca++]i from intracellular stores by stimulation of inositol-1,4,5-triphosphate receptor, and inducing insulin secretion. Other possible means of MTRs to regulate β-cell function are regulation of genes of the pancreatic circadian clock, or β-cell proliferation and differenciation. B, α-cells: In α-cells, a very similar granule exocytosis system to β-cells exists, based on K+ATP channels and L-type Ca++ channels. At low glucose concentrations, the system acts the same way as in β-cells: [Ca++]i increase leads to glucagon granule exocytosis and glucagon liberation. At high-glucose concentrations (high intracellular ATP), the closure of K+ATP channels leads to membrane hyperdepolarization, which triggers the shutting of Ca++ channels and inhibition of glucagon secretion. A cross talk between β- and α-cells is represented by IR activation in α-cells as an additional regulator with positive or negative effect of glucagon release, depending on the context. In addition, secreted glucagon is able to increase insulin secretion by activating the Gαs-coupled glucagon receptor (GcgR) present in ß-cells. Activated MTRs seem to act positively on granule secretion through a PLC/phosphatidyl-3-phosphate kinase (PI3K) pathway triggered by Gαq activation. Regulation of the pancreatic circadian clock is another putative way for MTRs to modulate α-cell function.