Abstract
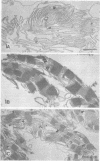
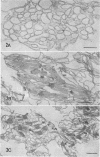
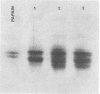
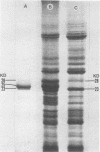
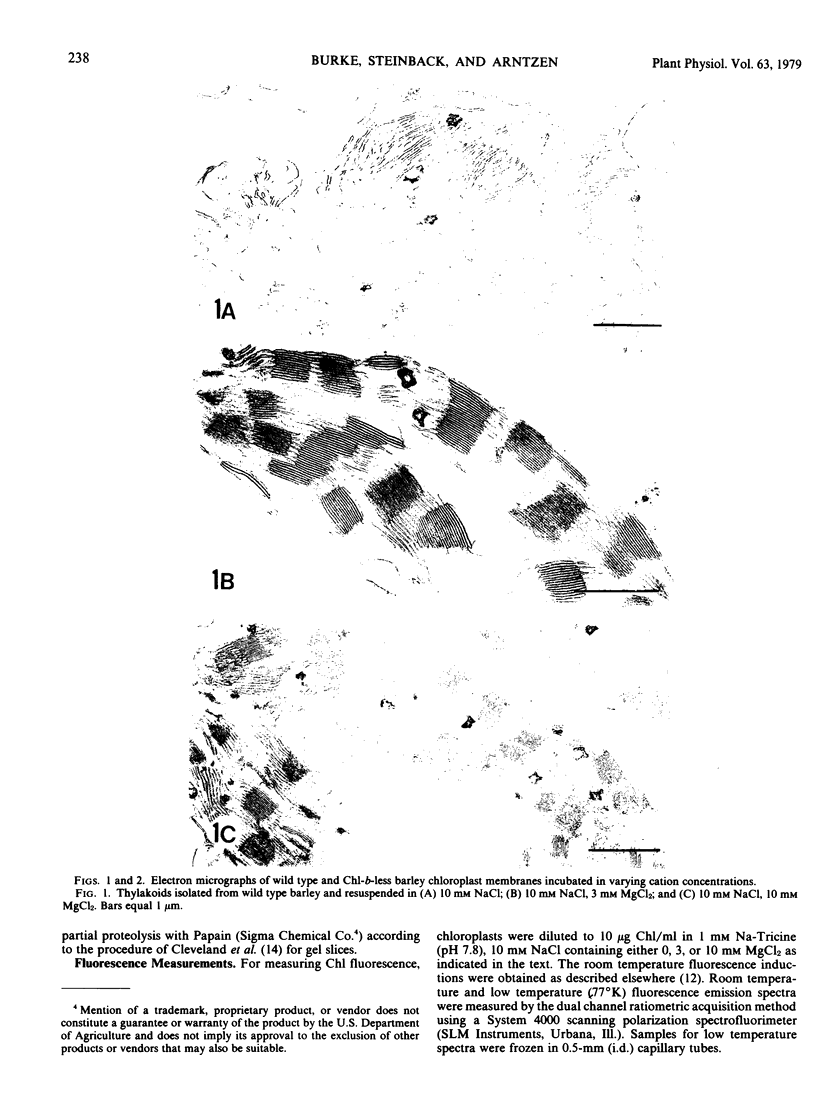
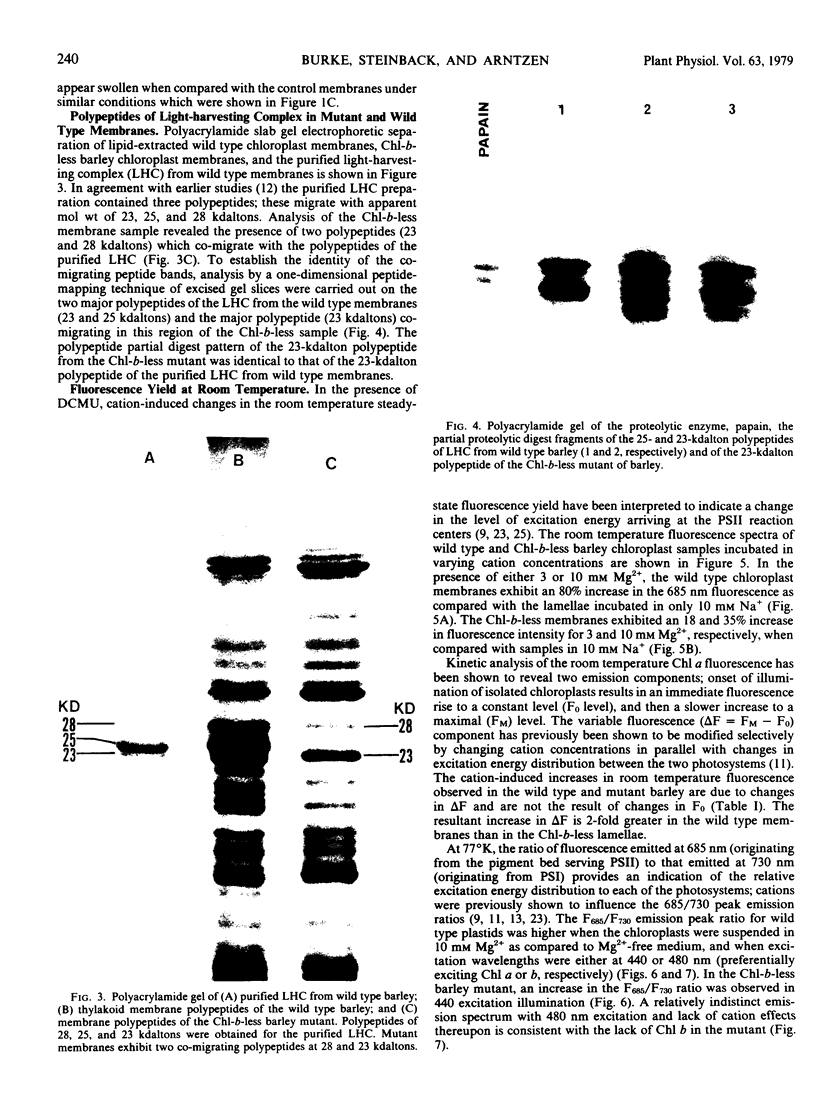
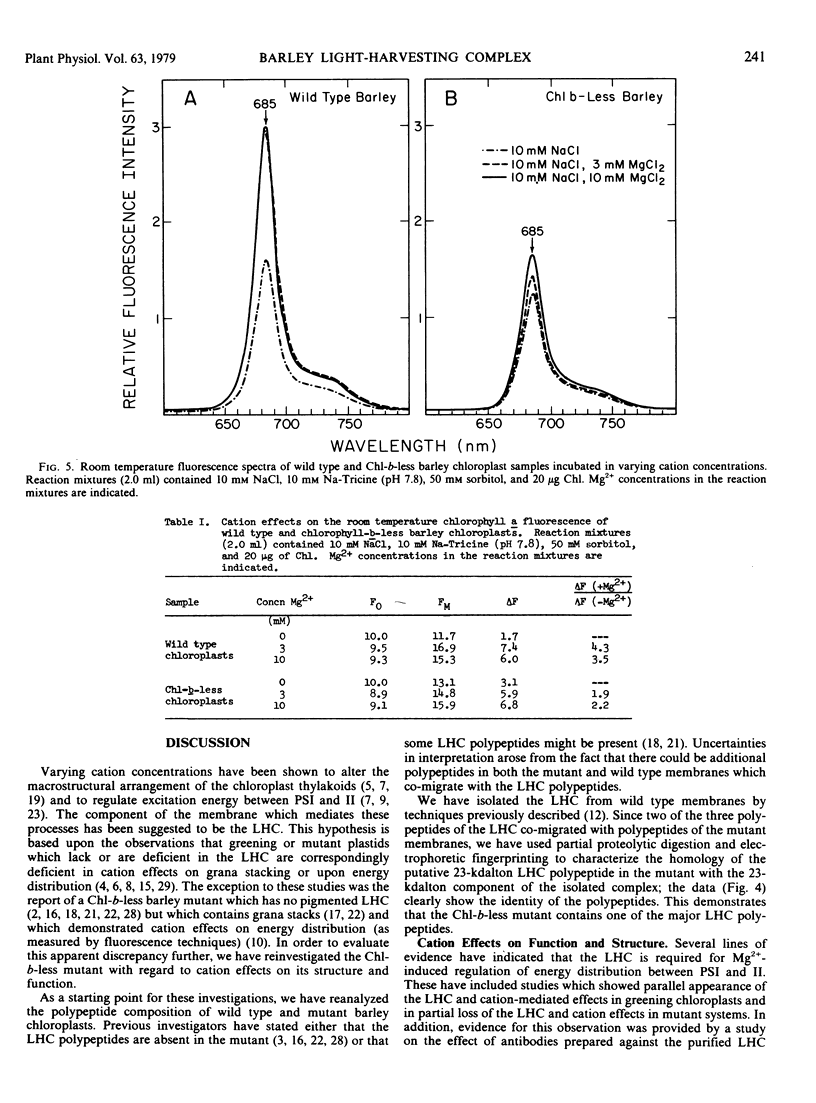
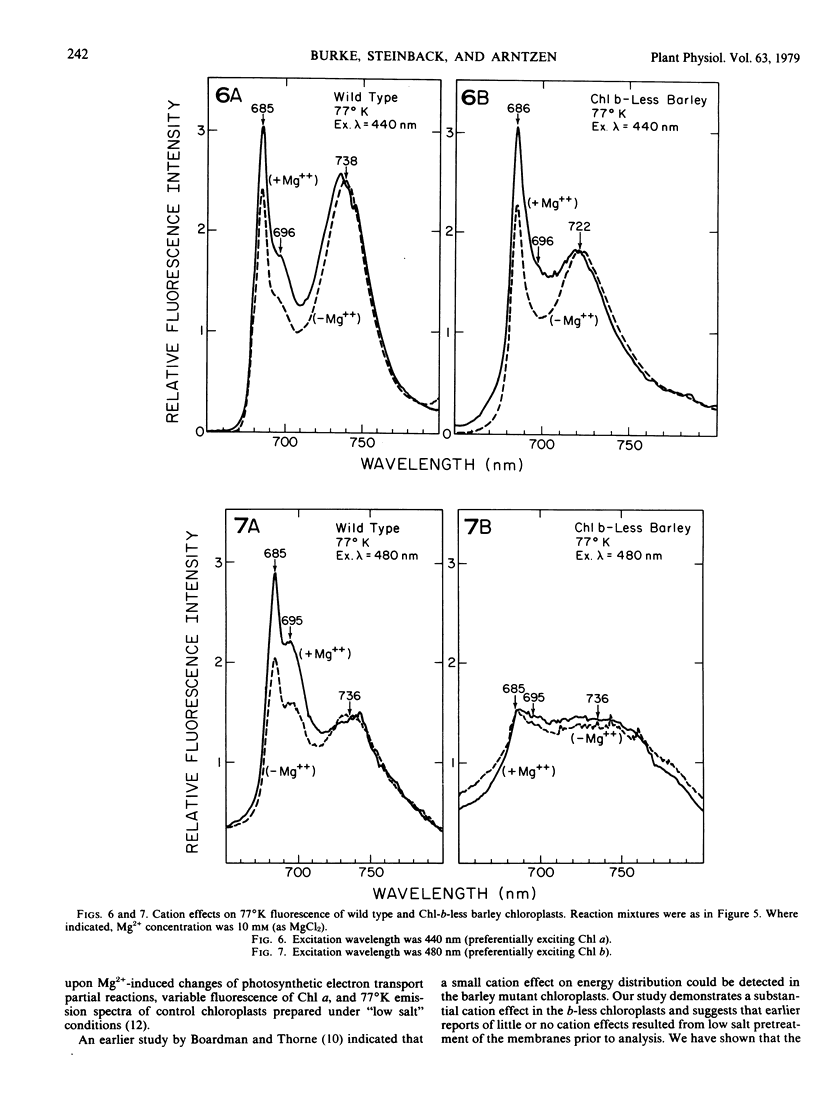
we have compared chloroplast lamellae isolated from a chlorophyll-b-less mutant and wild type barley (Hordeum vulgare). The results demonstrate that: (a) one of the two major polypeptides comprising the lightharvesting complex (LHC) is present in the chlorophyll-b-less mutant; (b) higher cation concentrations are required to maintain grana stacks in the mutant; and (c) cation effects on excitation energy distribution are present in the chlorophyll-b-less mutant but are reduced in amount and are dependent on higher concentrations of cations.
We interpret these data to support the concept that the LHC mediates cation-induced grana stacking and cation regulation of excitation energy distribution between photosystems I and Ii in chloroplast lamellae. A partial LHC complement in the mutant alters the quantitative cation requirement for both phenomena, but not the over-all qualitative response.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Levine R. P. The relationship between chlorophyll-protein complexes and chloroplast membrane polypeptides. Biochim Biophys Acta. 1974 Jul 25;357(1):118–126. doi: 10.1016/0005-2728(74)90117-0. [DOI] [PubMed] [Google Scholar]
- Anderson J. M. The molecular organization of chloroplast thylakoids. Biochim Biophys Acta. 1975 Aug 15;416(2):191–235. doi: 10.1016/0304-4173(75)90007-5. [DOI] [PubMed] [Google Scholar]
- Argyroudi-Akoyunoglou J. H., Akoyunoglou G. Correlation between cation-induced formation of heavy subchloroplast fractions and cation-induced increase in chlorophyll a fluorescence yield in tricine-washed chloroplasts. Arch Biochem Biophys. 1977 Mar;179(2):370–377. doi: 10.1016/0003-9861(77)90124-2. [DOI] [PubMed] [Google Scholar]
- Armond P. A., Arntzen C. J., Briantais J. M., Vernotte C. Differentiation of chloroplast lamellae. Light harvesting efficiency and grana development. Arch Biochem Biophys. 1976 Jul;175(1):54–63. doi: 10.1016/0003-9861(76)90484-7. [DOI] [PubMed] [Google Scholar]
- Briantais J. M., Vernotte C., Moya I. Intersystem excition transfer in isolated chloroplasts. Biochim Biophys Acta. 1973 Dec 14;325(3):530–538. doi: 10.1016/0005-2728(73)90212-0. [DOI] [PubMed] [Google Scholar]
- Burke J. J., Ditto C. L., Arntzen C. J. Involvement of the light-harvesting complex in cation regulation of excitation energy distribution in chloroplasts. Arch Biochem Biophys. 1978 Apr 15;187(1):252–263. doi: 10.1016/0003-9861(78)90031-0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Davis D. J., Armond P. A., Gross E. L., Arntzen C. J. Differentiation of chloroplast lamellae. Onset of cation regulation of excitation energy distribution. Arch Biochem Biophys. 1976 Jul;175(1):64–70. doi: 10.1016/0003-9861(76)90485-9. [DOI] [PubMed] [Google Scholar]
- Genge S., Pilger D., Hiller R. G. The relationship between chlorophyll b and pigment-protein complex II. Biochim Biophys Acta. 1974 Apr 23;347(1):22–30. doi: 10.1016/0005-2728(74)90196-0. [DOI] [PubMed] [Google Scholar]
- Goodchild D. J., Highkin H. R., Boardman N. K. The fine structure of chloroplasts in a barley mutant lacking chlorophyll B. Exp Cell Res. 1966 Oct;43(3):684–688. doi: 10.1016/0014-4827(66)90045-0. [DOI] [PubMed] [Google Scholar]
- Henriques F., Park R. B. Further Chemical and Morphological Characterization of Chloroplast Membranes from a Chlorophyll b-less Mutant of Hordeum vulgare. Plant Physiol. 1975 Apr;55(4):763–767. doi: 10.1104/pp.55.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa S., Good N. E. Effect of Salts and Electron Transport on the Conformation of Isolated Chloroplasts. II. Electron Microscopy. Plant Physiol. 1966 Mar;41(3):544–552. doi: 10.1104/pp.41.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller K. R., Miller G. J., McIntyre K. R. The light-harvesting chlorpohyll-protein complex of photosystem II. Its location in the photosynthetic membrane. J Cell Biol. 1976 Nov;71(2):624–638. doi: 10.1083/jcb.71.2.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N. Control of excitation transfer in photosynthesis. II. Magnesium ion-dependent distribution of excitation energy between two pigment systems in spinach chloroplasts. Biochim Biophys Acta. 1969 Oct 21;189(2):171–181. doi: 10.1016/0005-2728(69)90045-0. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Thornber J. P., Highkin H. R. Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem. 1974 Jan 3;41(1):109–116. doi: 10.1111/j.1432-1033.1974.tb03250.x. [DOI] [PubMed] [Google Scholar]