Fig. 4.
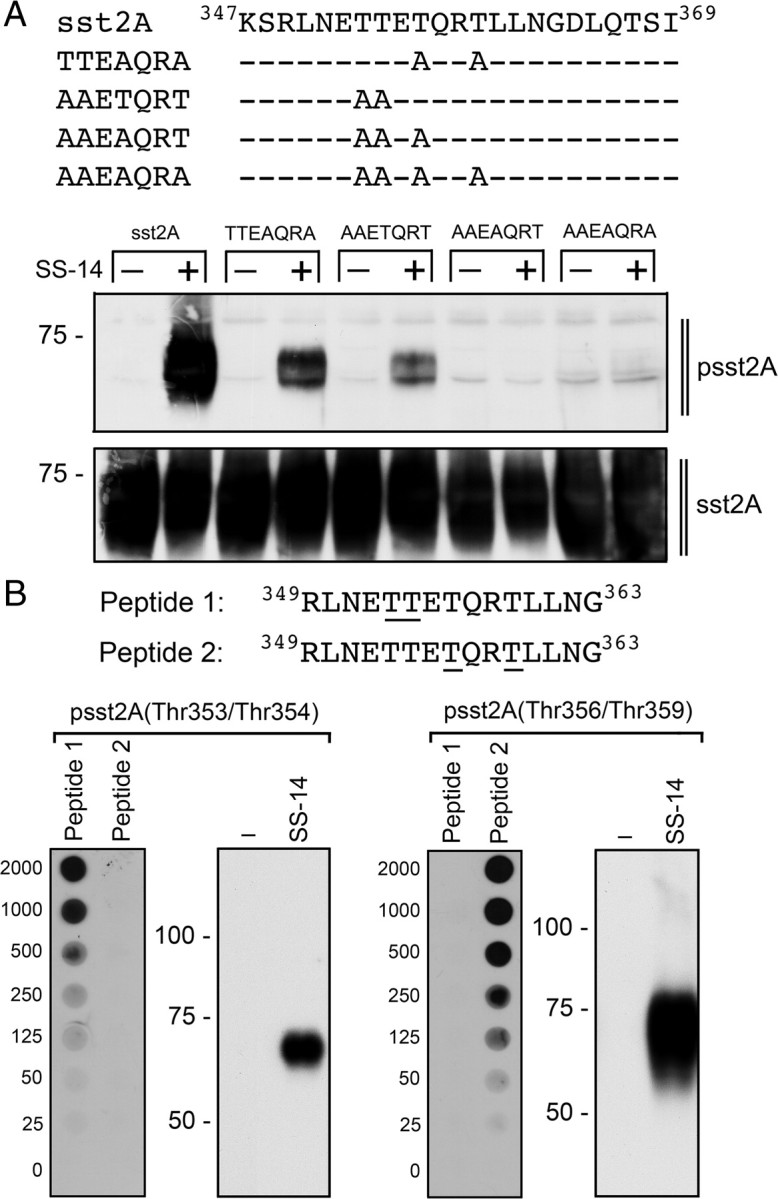
Mutational analysis of the carboxyl-terminal 353TTETQRT359 phosphorylation motif. A, Carboxyl-terminal amino acid sequences and site-directed mutants of sst2A (upper panel). HEK293 cells were stably transfected with wild-type sst2A, TTEAQRA, AAETQRT, AAEAQRT, or AAEAQRA and then treated with or without 1 μm SS-14 for 5 min (lower panel). The levels of phosphorylated sst2A receptors and total sst2A receptors were then determined by Western blot analysis. Note that the polyclonal anti-psst2A antiserum detects the phosphorylation of Thr353 and Thr354 in the TTEAQRA mutant as well as Thr356 and Thr359 in the AAETQRT mutant. B, Amino acid sequences of peptides used for immunoaffinity purification to produce anti-psst2A(Thr353/Thr354) or anti-psst2A(Thr356/Thr359) antibodies (upper panel). The underlined phosphate acceptor sites in peptide 1 and peptide 2 were phosphorylated. B, Characterization of anti-psst2A(Thr353/Thr354) and anti-psst2A(Thr356/Thr359) antibodies using Dot blot and Western blot analyses (lower panel). Dot blot: Serial dilutions (0–2000 ng) of peptide 1 and peptides 2 were blotted, and membranes were incubated with anti-psst2A(Thr353/Thr354) or anti-psst2A(Thr356/Thr359) antibodies. Western blot: HEK293 cells stably expressing sst2A were either not exposed or exposed to 1 μm SS-14 for 5 min. The levels of phosphorylated sst2A receptors were then determined using anti-psst2A(Thr353/Thr354) or anti-psst2A(Thr356/Thr359) antibodies. The positions of molecular mass markers are indicated on the left (in kilodaltons).
