Figure 2.
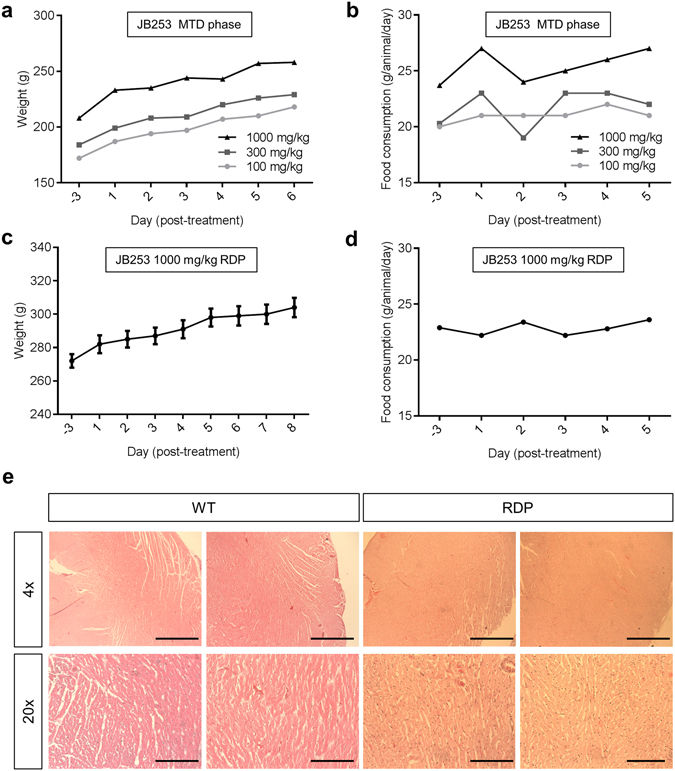
JB253 toxicology studies. (a) An animal receiving escalating doses (100–1000 mg/kg) during the maximum tolerated dose (MTD) phase did not show reductions in body weight). (b) As for (a), but food consumption (average chow consumed; n = 4 animals grouped). (c) As for (a), but showing no changes in weight during the repeated dose phase (RDP) at the maximum dose (1000 mg/kg) (n = 5 animals). (d) As for (b), but showing no changes in food consumption per animal during the RDP at the maximum dose (1000 mg/kg) (average chow consumed; n = 5 animals grouped). (e) Histopathological examination of hearts from wild-type (WT) and RDP animals was inconclusive with apparently normal cardiomyocyte morphology and no evidence of toxicity (representative images shown; n = 3–4 animals) (scale bar top = 500 µm; scale bar bottom = 100 µm). Animal numbers were set according to the UK Home Office, and in line with the OECD Mutual Acceptance of Data Agreement. Values represent mean ± SEM where grouped values are considered.
