Figure 3.
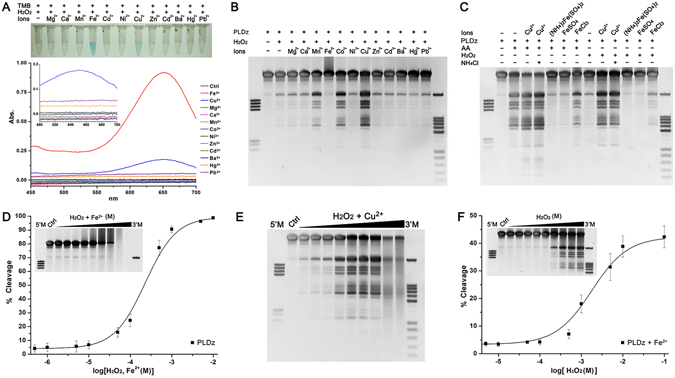
The role of hydroxyl radicals in the catalytic activity of PLDz. (A) Comparison of hydroxyl radical generation by treatment with different metal ions in the presence of H2O2. (B) Effects of divalent metal ions/H2O2 (100 μM) on the cleavage yield of PLDz. (C) Effects of Fe2+ (100 μM), Fe3+ (100 μM) and NH4 + (200 μM) on the cleavage yield of PLDz. (D) Effects of Fenton reagent (Fe2+/H2O2, 1 μM–0.01 M) on the cleavage yield of PLDz. (E) Effect of Cu2+/H2O2 concentration (1 μM–0.01 M) on the cleavage yield of PLDz. (F) Effect of H2O2 concentration (10 μM–0.1 M) on the cleavage yield of PLDz in present of 100 μM Fe2+. Reaction condition: 300 mM NaCl and 50 mM Tris-HCl (pH 7.0) at 23 °C for 2 hr. The error bars represented the standard deviations from three repeated measurements. Cropped gels are used in Fig. 3B–F, their full-length gels are presented in Supplementary Figure 8.
