Abstract
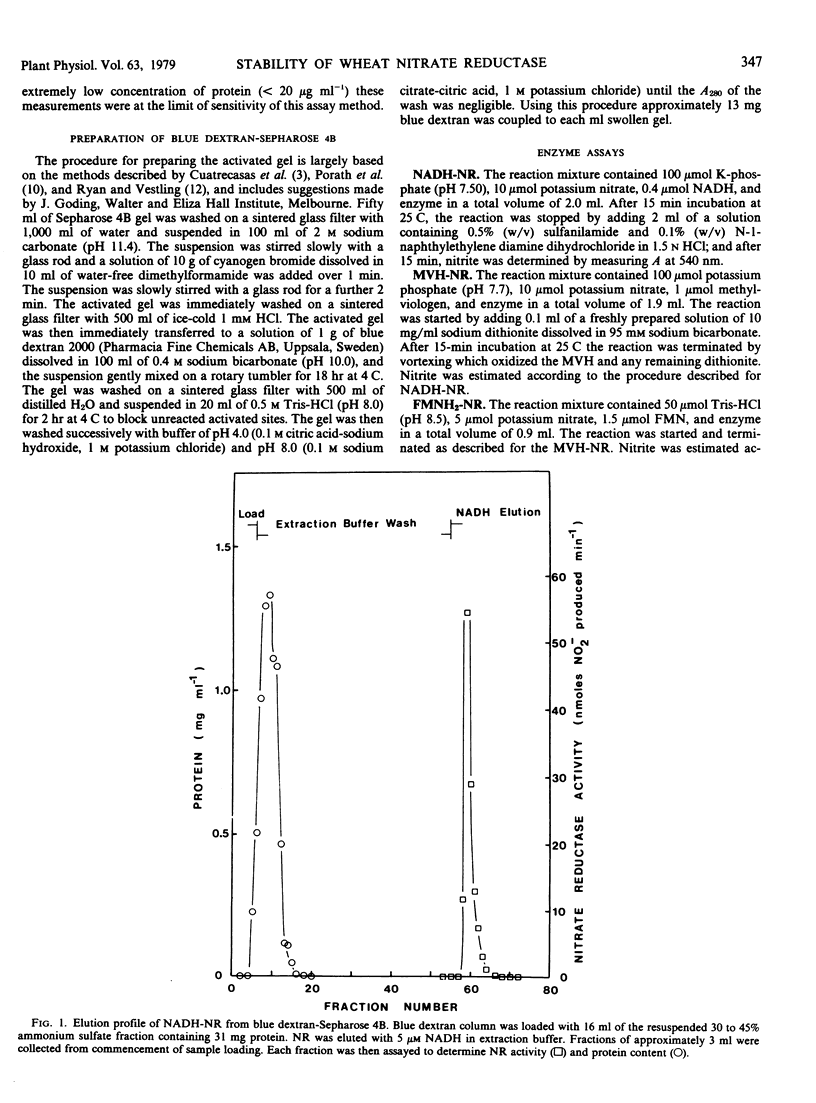
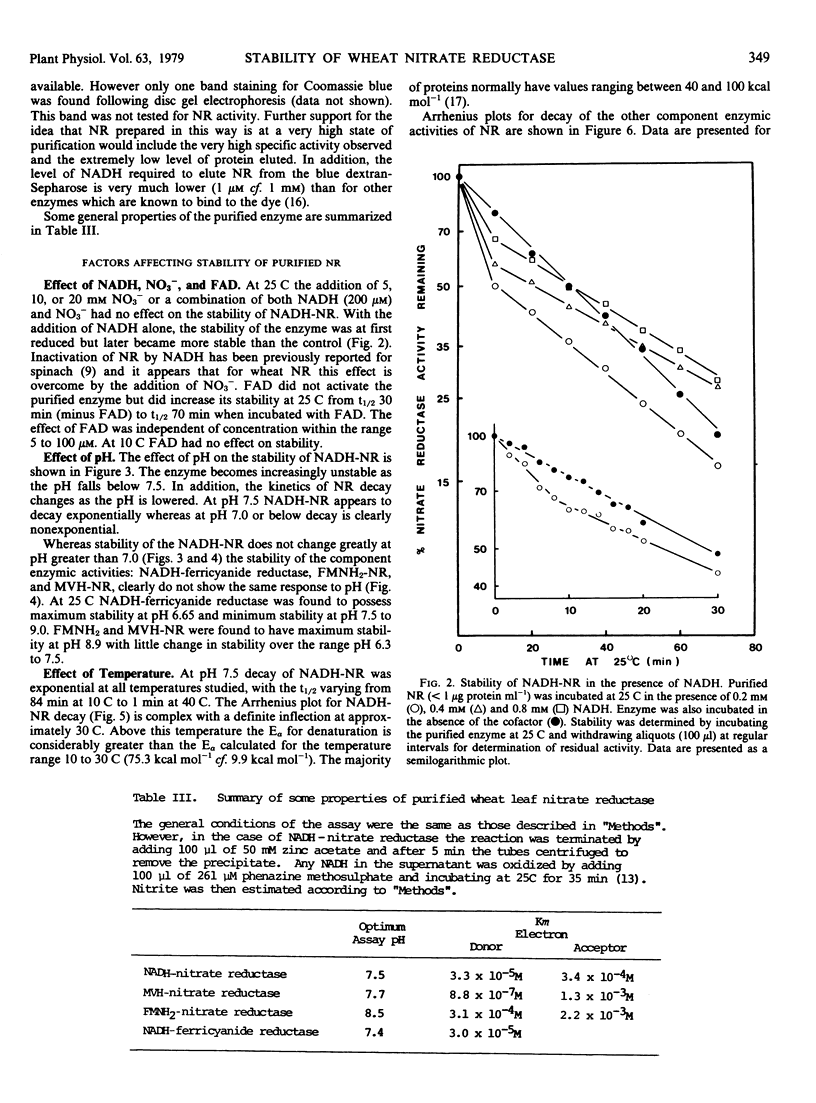
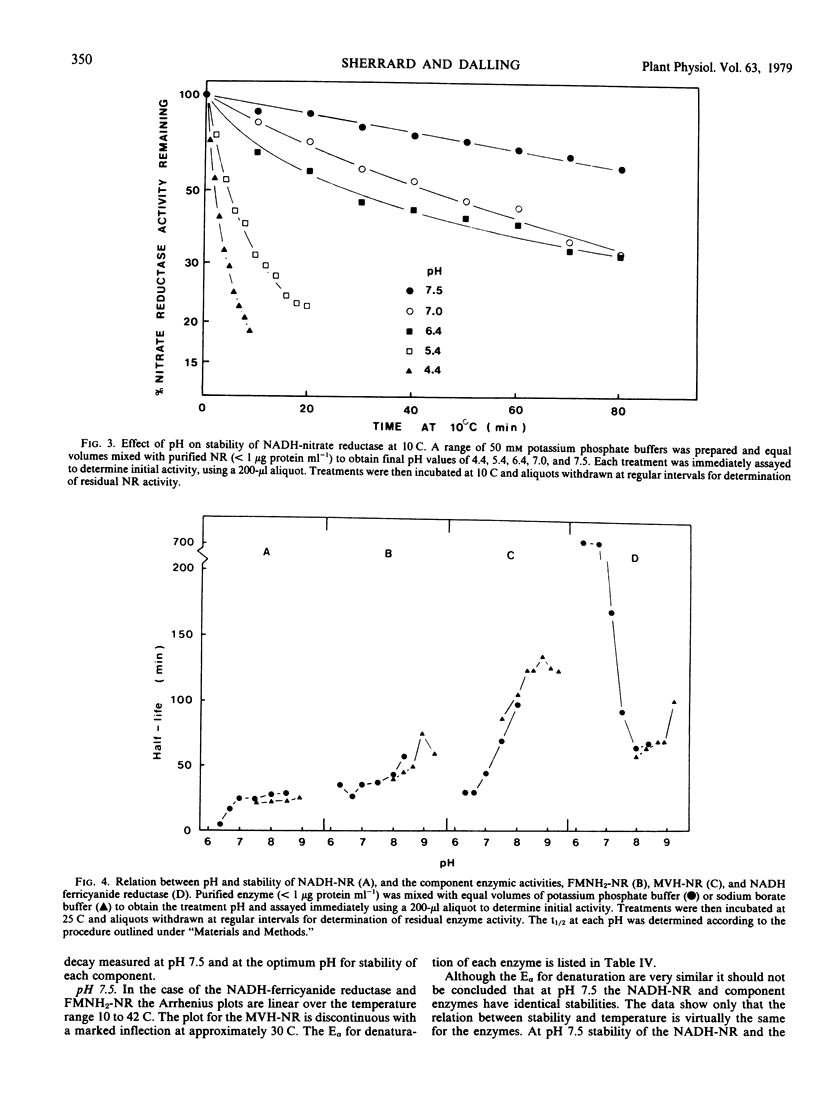
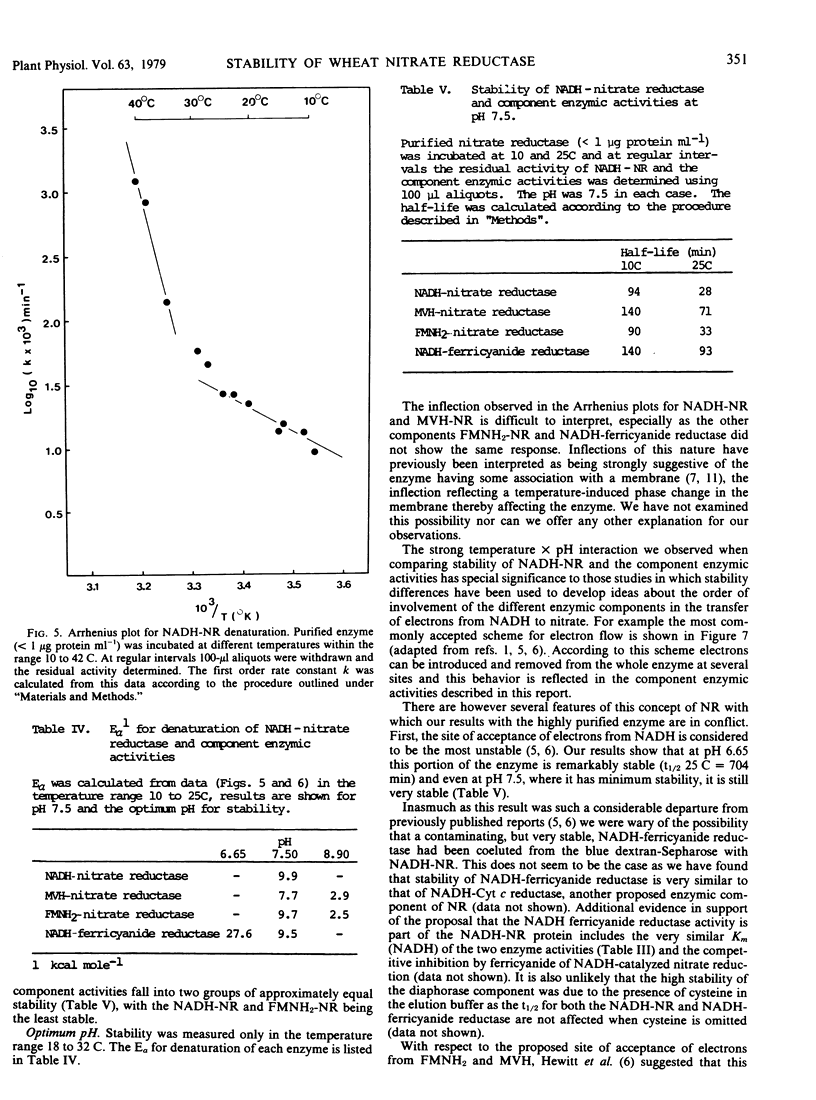
NADH-nitrate reductase has been highly purified from leaves of 8-day-old wheat (Triticum aestivum L. cv. Olympic) seedlings by affinity chromatography, using blue dextran-Sepharose 4B. Purification was assessed by polyacrylamide gel electrophoresis. The enzyme was isolated with a specific activity of 23 micromoles nitrite produced per minute per milligram protein at 25 C. At pH 7.5, the optimum pH for stability of NADH-nitrate reductase, this enzyme, and a component enzyme reduced flavin adenine mononucleotide (FMNH2)-nitrate reductase has a similar stability at both 10 and 25 C. Two other component enzymes—methylviologen-nitrate reductase and NADH-ferricyanide reductase—also have a similar but higher stability. At this pH the Arrhenius plot for decay of NADH-nitrate reductase and methylviologen-nitrate reductase indicates a transition temperature at approximately 30 C above which the energy of activation for denaturation increases. FMNH2-nitrate reductase and NADH-ferricyanide reductase do now show this transition. The energy of activation for denaturation (approximately 9 kcal per mole) of each enzyme is similar between 15 and 30 C. The optimum pH for stability of the component enzymes was: NADH-ferricyanide reductase, 6.6; FMNH2-nitrate reductase and methylviologen-nitrate reductase, 8.9. All of our studies indicate that the NADH-ferricyanide reductase was the most stable component of the purified nitrate reductase (at pH 6.6, t½ [25 C] = 704 minutes). Data are presented which suggest that methylviologen and FMNH2 do not donate electrons to the same site of the nitrate reductase protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K., Papahadjopoulos D. Effects of phospholipid acyl chain fluidity, phase transitions, and cholesterol on (Na+ + K+)-stimulated adenosine triphosphatase. J Biol Chem. 1974 Feb 25;249(4):1071–1080. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Palacián E., De la Rosa F., Castillo F., Gómez-Moreno C. Nitrate reductase from Spinacea oleracea. Reversible inactivation by NAD(P)H and by thiols. Arch Biochem Biophys. 1974 Apr 2;161(2):441–447. doi: 10.1016/0003-9861(74)90326-9. [DOI] [PubMed] [Google Scholar]
- Raison J. K., Lyons J. M., Thomson W. W. The influence of membranes on the temperature-induced changes in the kinetics of some respiratory enzymes of mitochondria. Arch Biochem Biophys. 1971 Jan;142(1):83–90. doi: 10.1016/0003-9861(71)90261-x. [DOI] [PubMed] [Google Scholar]
- Ryan L. D., Vestling C. S. Rapid purification of lactate dehydrogenase from rat liver and hepatoma: a new approach. Arch Biochem Biophys. 1974 Jan;160(1):279–284. doi: 10.1016/s0003-9861(74)80035-4. [DOI] [PubMed] [Google Scholar]
- Scholl R. L., Harper J. E., Hageman R. H. Improvements of the nitrite color development in assays of nitrate reductase by phenazine methosulfate and zinc acetate. Plant Physiol. 1974 Jun;53(6):825–828. doi: 10.1104/pp.53.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomonson L. P., Lorimer G. H., Hall R. L., Borchers R., Bailey J. L. Reduced nicotinamide adenine dinucleotide-nitrate reductase of Chlorella vulgaris. Purification, prosthetic groups, and molecular properties. J Biol Chem. 1975 Jun 10;250(11):4120–4127. [PubMed] [Google Scholar]
- Solomonson L. P. Purification of NADH-Nitrate Reductase by Affinity Chromatography. Plant Physiol. 1975 Dec;56(6):853–855. doi: 10.1104/pp.56.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. T., Cass K. H., Stellwagen E. Blue dextran-sepharose: an affinity column for the dinucleotide fold in proteins. Proc Natl Acad Sci U S A. 1975 Feb;72(2):669–672. doi: 10.1073/pnas.72.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]


