Abstract
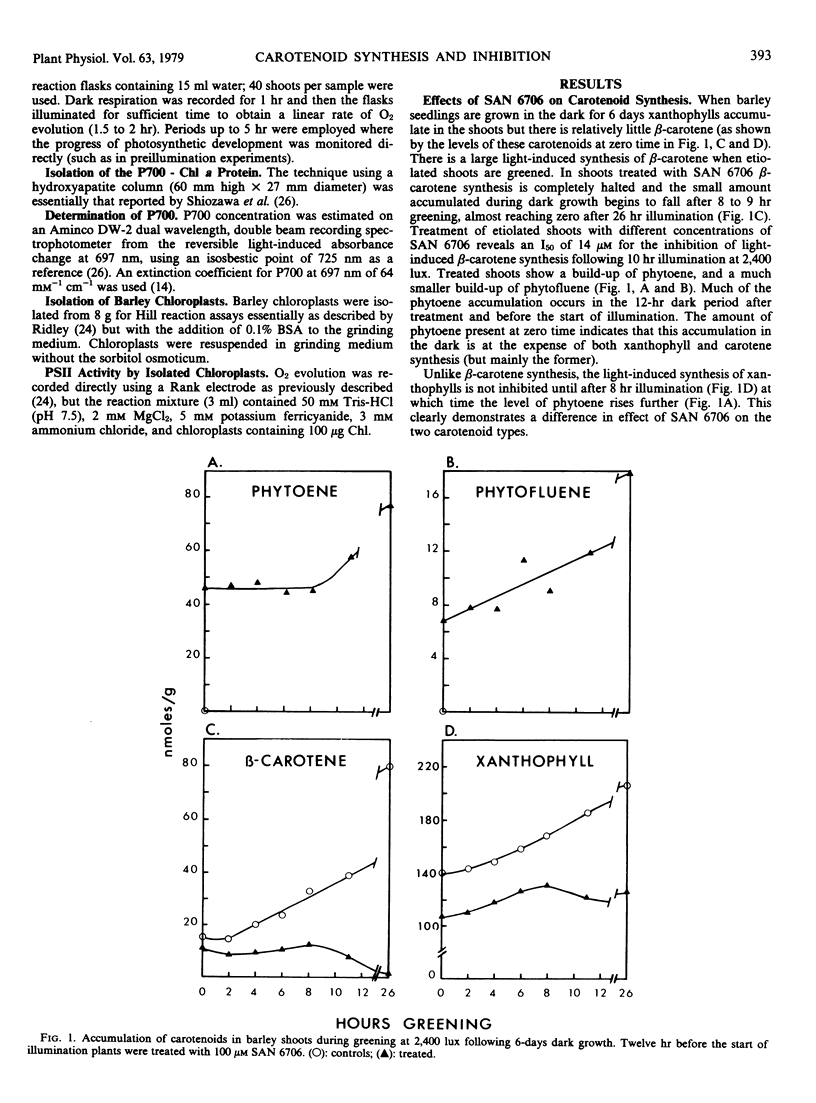
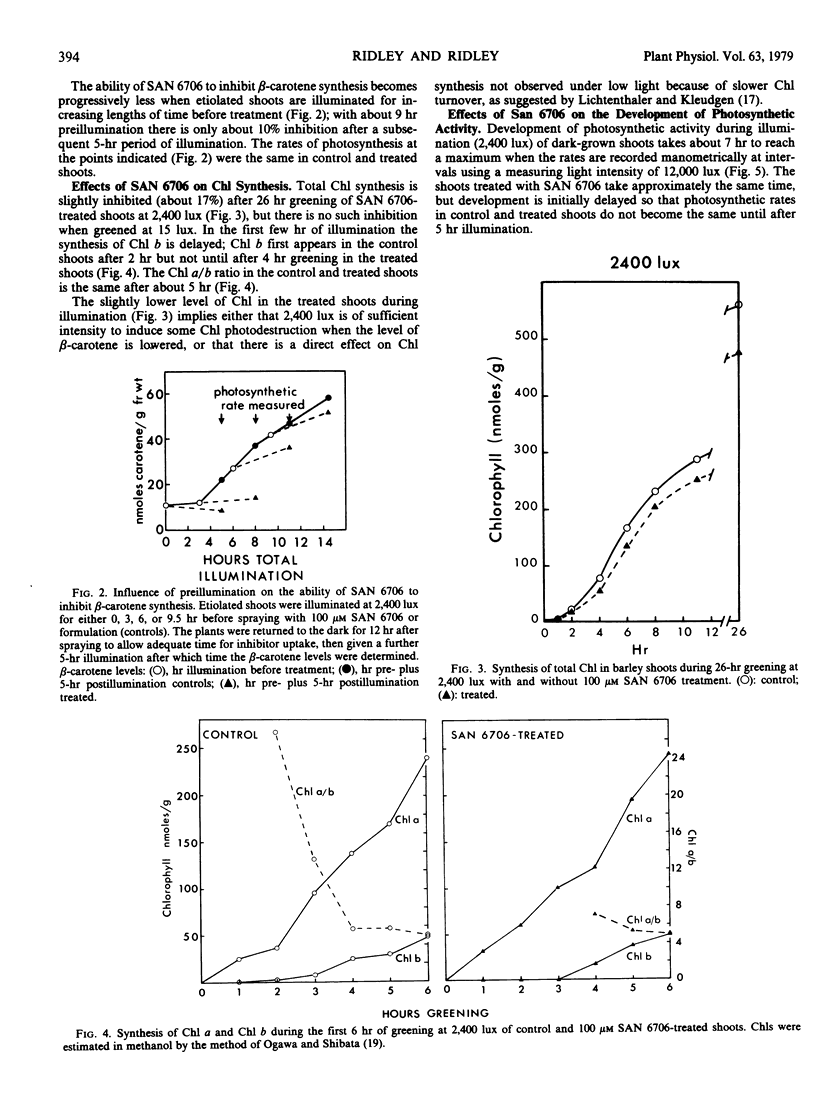
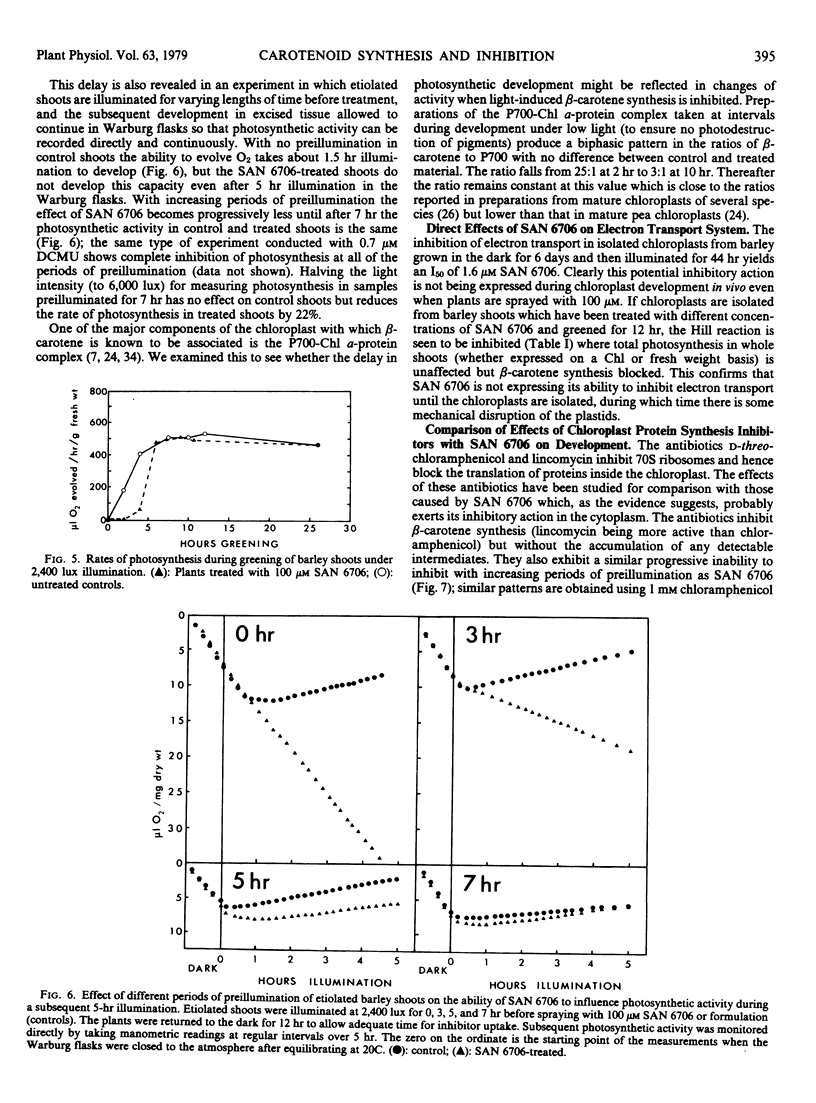
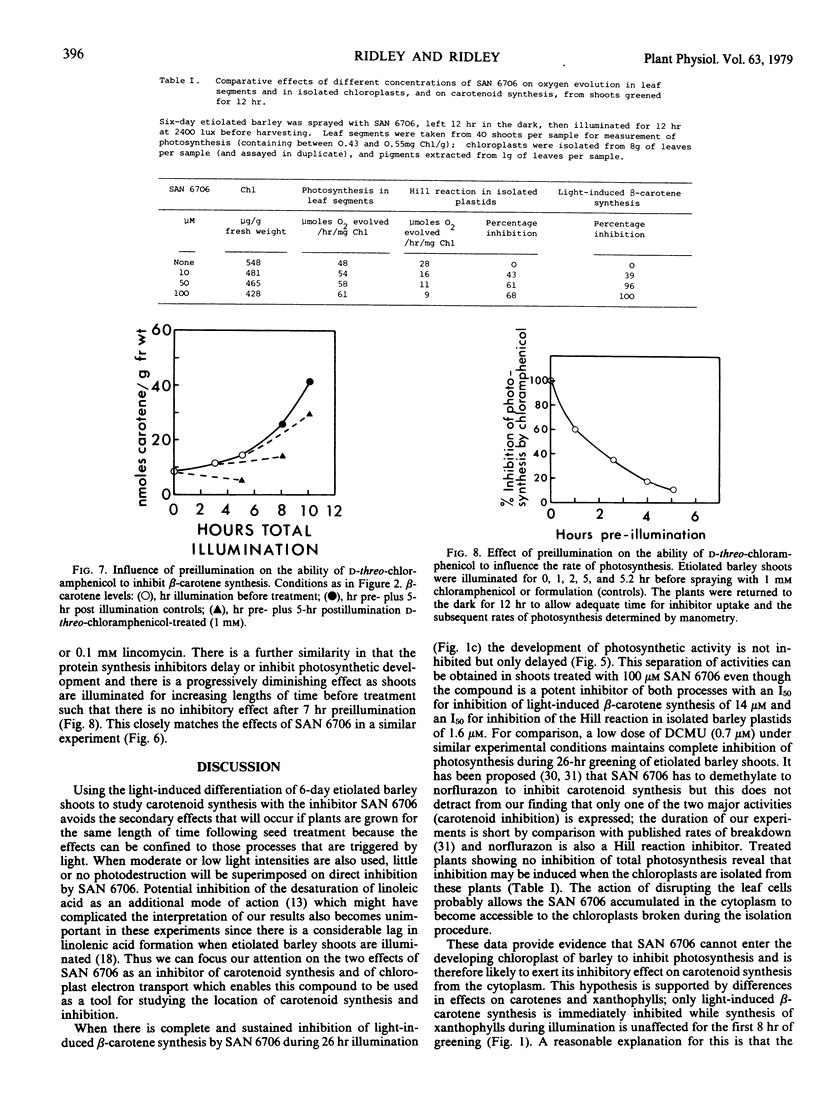
The inhibitor SAN 6706 [4-chloro-5-(dimethylamino)-2-(α,α,α,-trifluoro- m-tolyl-3(2H)-pyridazinone] has been used to study the synthesis of carotenes and xanthophylls during the conversion of etioplasts to chloroplasts in developing barley (Hordeum vulgare) shoots. SAN 6706 inhibits carotenoid synthesis and causes an accumulation of phytoene, but it is also a potent inhibitor of chloroplast electron transport. When developing barley is treated with SAN 6706, carotenoid synthesis is inhibited but total photosynthesis is unaffected. The ability of SAN 6706 to inhibit carotenoid synthesis becomes progressively less if etiolated shoots are illuminated for increasing lengths of time before treatment. During the greening of treated barley shoots only light-induced β-carotene synthesis is immediately inhibited; xanthophyll synthesis is not affected until after about 8 hours. The hypothesis that SAN 6706 cannot enter the chloroplast but inhibits carotenoid synthesis from the cytoplasm is discussed, and the question as to whether there are not two separate groups of enzymes for the synthesis of carotenes and xanthophylls is considered.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels P. G., Hyde A. Chloroplast Development in 4-Chloro-5-(dimethylamino)-2-(alpha,alpha,alpha-trifluoro-m-tolyl)-3 (2H)-pyridazinone (Sandoz 6706)-treated Wheat Seedlings: A Pigment, Ultrastructural, and Ultracentrifugal Study. Plant Physiol. 1970 Jun;45(6):807–810. doi: 10.1104/pp.45.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels P. G., McCullough C. A new inhibitor of carotenoid synthesis in higher plants: 4-chloro-5-(dimethylamino)-2- , , ,(trifluoro-m-tolyl)-3(2H)-pyridazinone. Biochem Biophys Res Commun. 1972 Jul 11;48(1):16–22. doi: 10.1016/0006-291x(72)90337-3. [DOI] [PubMed] [Google Scholar]
- Biggins J., Park R. B. CO(2) Assimilation by Etiolated Hordeum vulgare Seedlings during the Onset of Photosynthesis. Plant Physiol. 1966 Jan;41(1):115–118. doi: 10.1104/pp.41.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton J. L., John J. B., Christiansen M. N. Interactions of lipoidal materials and a pyridazinone inhibitor of chloroplast development. Plant Physiol. 1971 Aug;48(2):171–177. doi: 10.1104/pp.48.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama T., Ke B. Difference spectra and extinction coefficients of P 700 . Biochim Biophys Acta. 1972 Apr 20;267(1):160–171. doi: 10.1016/0005-2728(72)90147-8. [DOI] [PubMed] [Google Scholar]
- Newman D. W. The effect of red and far red light on the desaturation of Fatty acids in barley leaves. Plant Physiol. 1971 Sep;48(3):300–302. doi: 10.1104/pp.48.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesnicar M., Bendall D. S. The photochemical activities and electron carriers of developing barley leaves. Biochem J. 1973 Nov;136(3):803–812. doi: 10.1042/bj1360803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley S. M. Interaction of chloroplasts with inhibitors: induction of chlorosis by diuron during prolonged illumination in vitro. Plant Physiol. 1977 Apr;59(4):724–732. doi: 10.1104/pp.59.4.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa J. A., Alberte R. S., Thornber J. P. The P700-chlorophyll a-protein. Isolation and some characteristics of the complex in higher plants. Arch Biochem Biophys. 1974 Nov;165(1):388–397. doi: 10.1016/0003-9861(74)90177-5. [DOI] [PubMed] [Google Scholar]
- Smith J. H. The Development of Chlorophyll and Oxygen-evolving Power in Etiolated Barley Leaves When Illuminated. Plant Physiol. 1954 Mar;29(2):143–148. doi: 10.1104/pp.29.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang R. H., Rogers R. L. Behavior and fate of two phenylpyridazinone herbicides in cotton, corn, and soybean. J Agric Food Chem. 1974 Nov-Dec;22(6):1119–1125. doi: 10.1021/jf60196a051. [DOI] [PubMed] [Google Scholar]
- Subbarayan C., Kushwaha S. C., Suzue G., Porter J. W. Enzymatic conversion of isopentenyl pyrophosphate-4-14C and phytoene-14C to acyclic carotenes by an ammonium sulfate-precipitated spinach enzyme system. Arch Biochem Biophys. 1970 Apr;137(2):547–557. doi: 10.1016/0003-9861(70)90472-8. [DOI] [PubMed] [Google Scholar]
- Thornber J. P., Stewart J. C., Hatton M. W., Bailey J. L. Studies on the nature of chloroplast lamellae. II. Chemical composition and further physical properties of two chlorophyll-protein complexes. Biochemistry. 1967 Jul;6(7):2006–2014. doi: 10.1021/bi00859a019. [DOI] [PubMed] [Google Scholar]
- Vaisberg A. J., Schiff J. A. Events Surrounding the Early Development of Euglena Chloroplasts: 7. Inhibition of Carotenoid Biosynthesis by the Herbicide SAN 9789 (4-Chloro-5-(methylamino)-2-(alpha,alpha,alpha,-trifluoro-m-tolyl)-3-(2H)pyridazinone) and Its Developmental Consequences. Plant Physiol. 1976 Feb;57(2):260–269. doi: 10.1104/pp.57.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisberg A. J., Schiff J. A., Li L., Freedman Z. Events Surrounding the Early Development of Euglena Chloroplasts: VII. Photocontrol of the Source Of Reducing Power for Chloramphenicol Reduction by the Ferredoxin-NADP Reductase System. Plant Physiol. 1976 Apr;57(4):594–601. doi: 10.1104/pp.57.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]


