Figure 3.
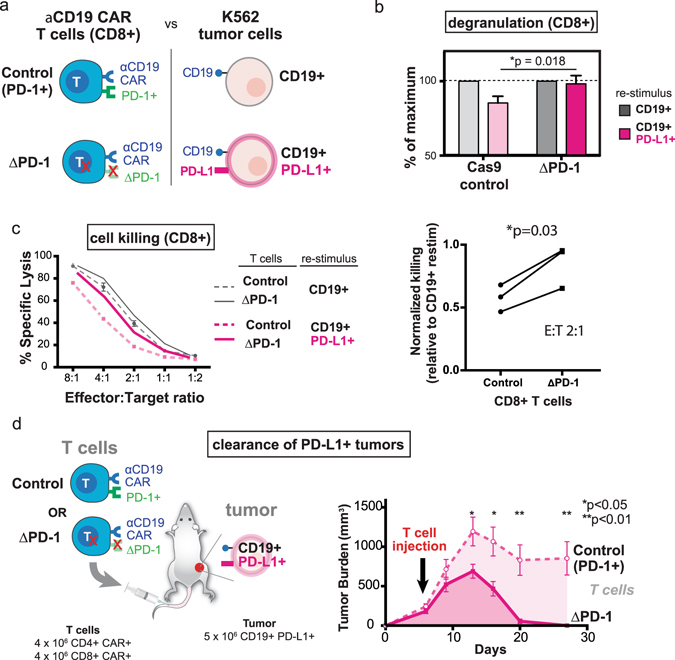
CRISPR-mediated PD-1 editing rescues anti-CD19 CAR T cell function in vitro and enhances tumor clearance in vivo. (a) Diagram of PD-1 edited CAR T cell: K562 interactions (b) PD-1 edited CD8+ anti-CD19 CAR T cells (ΔPD-1) exhibit greater degranulation (CD107a staining) upon co-culture with CD19+ PD-L1+ K562 cells as compared to control CD8+ CAR T cells (*p = 0.018, Student’s t-test). (c) PD-1 edited CAR T cells are partially resistant to CD19+ PD-L1+ mediated inhibition of cytolysis. Left panel: percent lysis for control and PD-1 edited CD8+ anti-CD19 CAR T cells is shown across a range of effector:target ratios. Error bars are S.D. of triplicate wells in a single experiment. Right panel: normalized killing shows reduced PD-L1 dependent inhibition of killing in PD-1 edited CD8+ CAR T cells at effector:target ratio of 2:1 (*p = 0.03, paired t-test). The experiment was performed three independent times. (d) PD-1 deficient anti-CD19 CAR T cells exhibit enhanced anti-tumor efficacy and clear subcutaneous CD19+ PD-L1+ tumor xenografts. NSG mice were injected with 5 × 106 CD19+ PD-L1+ K562 cells subcutaneously. Mice with established tumors (100–250 mm3) were injected intravenously with 4 × 106 CD4+ CAR+ and 4 × 106 CD8+ CAR+ control T cells or PD-1 edited cells, and tumor burden measured longitudinally by caliper. Tumor burdens are mean ± SEM for each group (n = 6 mice per group). A statistically significant decrease in tumor burden of mice receiving PD-1 edited CAR T cells was observed at multiple points (*p < 0.05, **p < 0.01, Student’s t-test).
