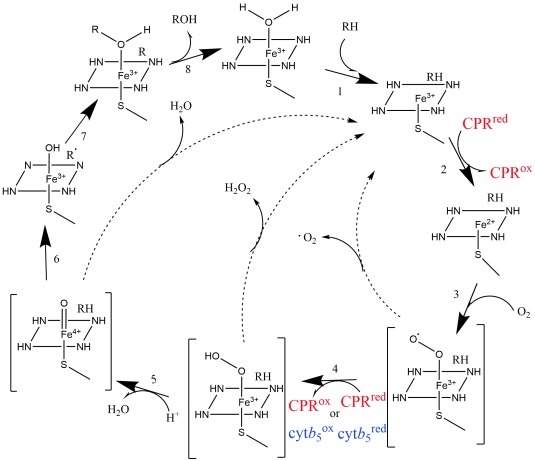
Figure 1. The catalytic cycle of cytochrome P450.
The binding of substrate R-H (1) causes a decrease of the redox potential of about 100 mV, which allows the first electron transfer from cytochrome P450-reductase (CPR) (2). The reduction of Fe 3+ to Fe 2+ makes suitable O 2 binding (3), which now can accept a second electron from either CPR or cytochrome b 5 (cyt b 5) (4), to form a hydroperoxyl intermediate known as compound 0. The O 2 −2 complex reacts with surrounding protons to form the highly reactive oxyferryl intermediate, also known as compound I (5). The Fe-ligated O atom (6) is transferred to the substrate forming a hydroxylated form of the substrate (7). The product is finally released (8), replaced by a molecule of water. Three uncoupling reactions are shown as dashed lines, with the respective products: the autoxidation shunt O 2 −2, the peroxide shunt (H 2O 2), and the oxidase shunt (H 2O).