Figure 3.
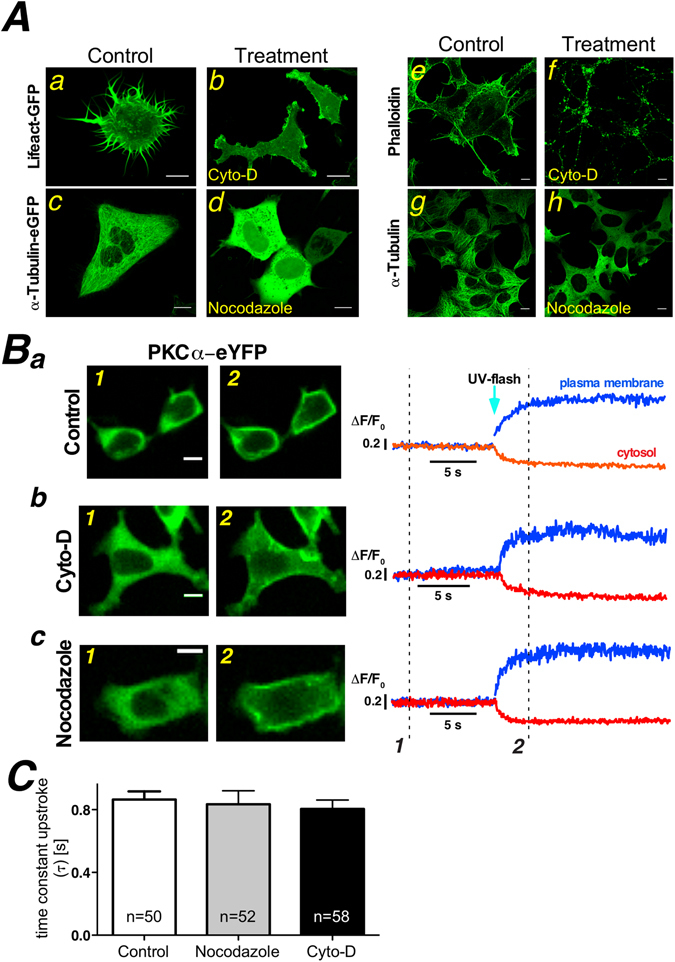
Depolymerization of actin filaments and microtubules does not alter Ca2+ induced PKCα translocation. (A) Cytochalasin-D (4 µM, 2 hours) (b,f) or nocodazole (10 µM, 2 hours) (d,h) treatment of HEK cells, either expressing Lifeact-GFP (a,b) and α-tubulin-eGFP (c,d), staining with phalloidin (e,f) or immunofluorescence with primary antibodies against α-tubulin (g,h), results in depolymerisation of actin filaments (b,f) and microtubules (d,h), respectively. Note that the confocal sections in Aa and Ab were deliberately close to the bottom of the cell to highlight the spiky plasma membrane protrusion while all other confocal sections were placed in the middle of the nucleus. (B) Exemplified single images of PKCα-eYFP distribution before (Ba1, Bb1, Bc1) and following (Ba2, Bb2, Bc2) photolytic Ca2+ increase at the time points marked in the traces to the right. Three different experimental conditions are depicted (Ba-control, Bb-cytochalasin-D treatment, Bc- nocodazole treatment). Traces were generated from regions of interest in the cytosol (red traces) and on the plasma membrane (blue traces). (C) The plasma membrane accumulation was characterized by fitting an exponential to the upstroke following the flash photolytic Ca2+ increase. The statistical summary demonstrates that the state of polymerization of the cytoskeleton does not influence the speed at which PKCα-eYFP accumulates at the plasma membrane after Ca2+ UV flash photolysis. Numbers given on the bars indicate number of cells in at least 5 independent experiments.
