Supplemental Digital Content is available in the text
Keywords: HIV, MRS, PCNSL, PET, SPECT
Abstract
Background:
We performed a systematic review and meta-analysis to assess the roles of SPECT, PET, and MRS in distinguishing primary central nervous system lymphoma (PCNSL) from other focal brain lesions (FBLs) in human immunodeficiency virus (HIV)-infected patients.
Methods:
PubMed, Scopus, and Medline were systematically searched for eligible studies from 1980 to 2016. Two authors extracted characteristics of patients and their lesions using predefined criteria.
Results:
Eighteen studies on SPECT containing 667 patients, 6 studies on PET containing 108 patients, and 3 studies on MRS containing 96 patients were included. SPECT had a pooled sensitivity of 0.92 (95% CI: 0.85–0.96) and specificity of 0.84 (95% CI: 0.74–0.90) in differentiating PCNSL from other FBLs. For the 6 studies that used only pathology and/or serology as the gold standard, the pooled sensitivity was 0.85 (95% CI: 0.72–0.97) and the pooled specificity was 0.73 (95% CI: 0.54–0.92).
Conclusion:
SPECT has good diagnostic accuracy for discriminating PCNSL from other FBL-causing disorders in HIV patients. However, the actual sensitivity and specificity of SPECT may be lower than expected if only pathology and/or serology was used as the gold standard. PET may be superior but has less supporting clinical data and is more expensive.
1. Introduction
Neurological complications affect 40% to 80% of human immunodeficiency virus (HIV)-infected patients.[1] CNS-related pathology mainly results from opportunistic infections such as toxoplasmosis, cytomegalovirus, and progressive multifocal leukoencephalopathy.[2] Primary central nervous system lymphoma (PCNSL) is also an important etiology of focal brain lesions (FBLs) in HIV-infected individuals with a reported incidence of 2% to 6%, at least 1000 times higher than that in the general population.[3] Despite advances in new treatment strategies, the median survival of HIV patients with PCNSL not receiving radiation or other targeted therapies immediately after the onset of clinical symptoms is 1 month.[4] Due to the rapid progression of the disease, a fast and reliable diagnosis is essential.
It is often difficult to differentiate PCNSL from other FBLs, particularly from toxoplasmosis in HIV patients. The 2 diseases share similar clinical symptoms and demonstrate nearly identical imaging features on routine CT and MRI.[5–10] In these patients, it is standard practice to begin empirical antitoxoplasmosis treatment.[11] Brain biopsy is usually performed when treatments fail to lead to clinical and radiological improvements. Brain biopsy, despite being the current diagnostic gold standard, is invasive and has high morbidity.[12] Over the past few decades, noninvasive functional nuclear imaging modalities such as single-photon emission computed tomography (SPECT) and positron emission tomography (PET) have been used to detect PCNSL based on physiologic differences between lymphoma and infectious lesions. Several studies have shown high diagnostic accuracy of both SPECT[10,13–17] and PET,[18–23] but a few other studies have cast doubt on the utility of SPECT.[5,24,25] Advanced MRI techniques, including apparent diffusion coefficient (ADC) ratios,[26,27] regional cerebral blood volume (rCBV),[28] and magnetic resonance spectroscopy (MRS),[29–35] have demonstrated promise in differentiating PCNSL from other FBLs, but the results are variable. Due to the small number of studies and limited cohort sizes of existing studies, it is challenging to create guidelines for clinical practice based on published literature.
The main aim of this study is to perform a systematic review and meta-analysis of the published studies to assess the diagnostic accuracy of SPECT, PET, and MRS in differentiating PCNSL from other FBLs in HIV patients.
2. Methods
No institutional review board approval was required for this study. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed.[36] The electronic databases used were PubMed, Scopus, and Medline. The keywords used were a combination of “HIV” or “AIDS,” “lymphoma,” “brain,” “SPECT” or “PET” or “MRI,” “toxoplasmosis,” and their variations. Reference lists of the included papers were reviewed to identify potential papers. Only studies published from 1980 to 2016 in English were included.
Two authors (MY and HXB) independently examined the titles and abstracts or full texts when necessary to determine eligible studies. In case of disagreement or uncertainty, a consensus was reached by consulting with a third author (LY). Inclusion criteria were: All patients included in the study were infected with HIV; All patients had FBLs on MRI and/or CT before SPECT, PET, or MRS was performed; The study contained data that could be converted to analyze diagnostic accuracy (ie, 2 × 2 table); and Final diagnosis was clearly stated in the paper. Exclusion criteria were: Cohort size was too small (<10); The patient cohort in the study overlapped with that in a previous study; The study focused on imaging modalities other than SPECT, PET, or MRS; and The study was in the format of letters, comments, case reports, or personal communications.
Two authors (MY and HBX) extracted data together from the 1st 5 articles and then individually for the rest of the articles. The variables extracted included: characteristics of the studies such as name of the first author, year of publication, country in which the study was conducted, study design (retrospective or prospective), and study size; patient characteristics such as age, gender, and final diagnoses of the nonlymphoma group; lesion characteristics such as the number of lesions in the brain and lesion size; imaging techniques such as modality and tracer used; analytical method (visual evaluation vs quantitative analysis); reference standard (pathological or serological diagnosis vs clinical follow-up); and average follow-up time of patients.
Two authors (MY and HBX) assessed the methodological quality of the selected studies using Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2. Discrepancies were resolved by consensus meetings in a panel including a 3rd author (LY). The risk of bias was analyzed in 4 domains: patient selection, index test, reference standard, and flow and timing. Applicability was assessed in the following 3 domains: patient selection, index test, and reference standard. Signaling questions were applied to determine the risk of bias and applicability.
We included prospective and retrospective studies of HIV patients with FBLs who had SPECT, PET, or MRS scans. We calculated the pooled sensitivity and specificity, likelihood ratio (LR), and the area under the receiver-operating characteristic curve (AUC). Hierarchical summary receiver-operating characteristic (HSROC) curve was constructed for SPECT. A 2-sided P value less than .05 was considered statistically significant. The nonthreshold effect that contributed to heterogeneity was assessed by chi-square (χ2) and inconsistency index (I2) statistics. If I2 > 50%, a random-effects model was used, and a fixed-effects model was used otherwise. Publication bias was evaluated by the Deek test for funnel plot asymmetry. Sensitivity analysis was conducted using the leave-one-out approach. In case of significant heterogeneity, subgroup analysis via meta-regression was performed to explore a potential source of heterogeneity by calculating the I2 statistics. The covariates investigated included study design, study size, geographical region, tracer applied, reference standard, analytical method, and percent of nonlymphoma final diagnoses which were toxoplasma encephalitis (TE). All analyses were performed using StataIC 14 (StataCorp, College Station, TX).
3. Results
We screened 5248 articles initially for potentially eligible studies (Fig. 1). Per inclusion criteria, 82 studies remained. After full review of the 82 papers, 59 studies were excluded. We included 3 additional studies by reviewing the reference lists of the 82 papers. A total of 26 studies were included in the final analysis: 18 studies on SPECT containing 667 patients, 6 studies on PET containing 108 patients, and 3 studies on MRS containing 96 patients. One study included both PET and MRS.[37]
Figure 1.
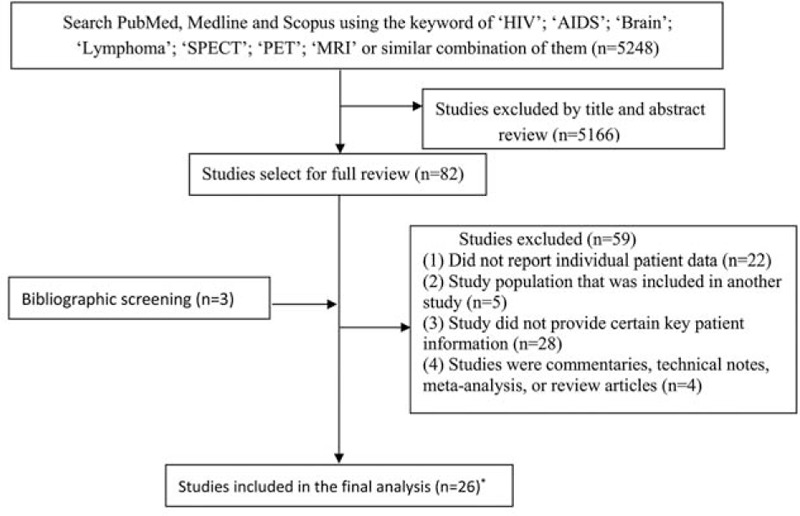
Flow chart for the identification of eligible studies. ∗Westwood et al studied both PET and MRS. MRS = magnetic resonance spectroscopy, PET = positron emission tomography.
The included studies were published between 1994 and 2016. Thirteen studies were from the US, 11 were from Europe, and 2 were from Asia. The average age of all included patients was 36.9 (range: 16–79). For SPECT studies, Thallium-201 was used as the only tracer in 14 studies and Technetium-99m sestamibi as the only tracer in 1. Three studies used a combination of different tracers, which were counted separately in our meta-analysis. For PET studies, all 6 studies used 18F-fluorodeoxyglucose as the only tracer. For SPECT, 5 studies used only pathology and/or serology as the reference standard for the final diagnosis, while 13 studies used clinical follow-up as the reference standard in addition to pathology and/or serology. For PET and MRS, all 6 PET studies and 3 MRS studies used a combination of pathology, serology, and clinical follow-up as the reference standard. Among the 17 studies on SPECT which provided information on method of analysis, quantitative analysis was performed in 10 studies, while qualitative analysis was applied in 7 studies. For PET, quantitative analysis was performed in 3 studies, while visual inspection was used in the other 3 studies. For MRS, all 3 studies used quantitative analysis. In all 3 MRS studies, NAA/Cr, NA/Cr, Cho/Cr, and NAA/Cho ratios were used as parameters to estimate the diagnostic accuracy of MRS. Among all studies, only 3 reported mean single lesion size.[14,24,38] Shyam Babu et al separated all lesions into those larger than 1 cm and smaller than 1 cm.[5] Characteristics of included studies on SPECT, PET, and MRS are summarized in Supplemental Tables 1 to 3.
Seven domains were assessed regarding bias and applicability for each paper. Out of the 182 domains in total including all papers, 23 domains were determined as high risk (15 for risk of bias and 8 for applicability concerns). Therefore, the overall quality was acceptable. We found that the bias stemmed mainly from the index test (n = 6), flow and timing (n = 6), and patient selection (n = 11) domains. The quality assessment of the 26 included papers using QUADAS-2 is shown in Supplemental Table 4.
The forest plot of sensitivity and specificity for individual SPECT studies is shown in Fig. 2. The I2 values for sensitivity and specificity of the 18 included studies were 52.1 (95% CI: 28.3–76.0) and 76.1 (95% CI: 66.1–86.1), respectively. A random-effect model was used to calculate pooled sensitivity and specificity, which were 0.92 (95% CI: 0.85–0.96) and 0.84 (95% CI: 0.74–0.90), respectively (Fig. 2). The overall LR+ and LR− for SPECT were 5.6 (95% CI: 3.5–9.0) and 0.10 (95% CI: 0.05–0.19). The HSROC curve is shown in Fig. 3 with an area under curve (AUC) of 0.95. Pooled diagnostic odds ratio (DOR) of the 18 studies was 57 (95% CI: 25–131).
Figure 2.
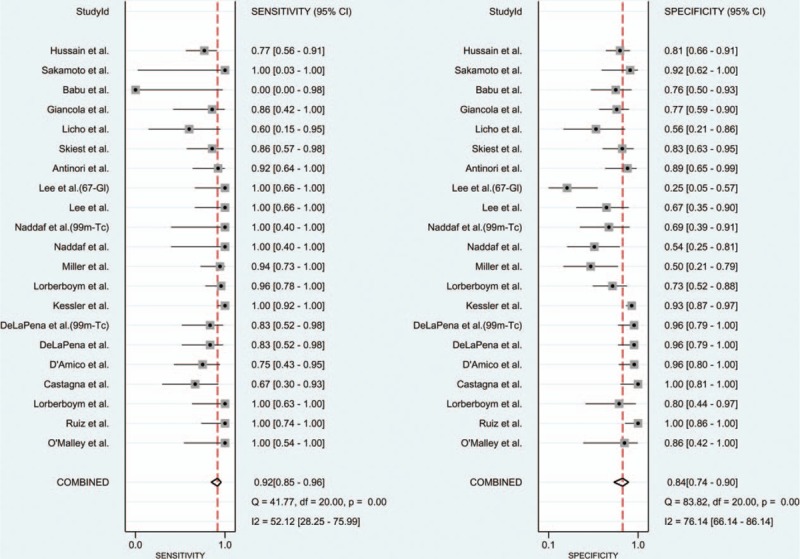
Forest plot of sensitivity and specificity for SPECT. All tracers used were thallium-201 chlorideunless specifically labeled. 67-Gl = gallium-67, 99m-Tc = technetium sestamibi, SPECT = single-photon emission computed tomography.
Figure 3.
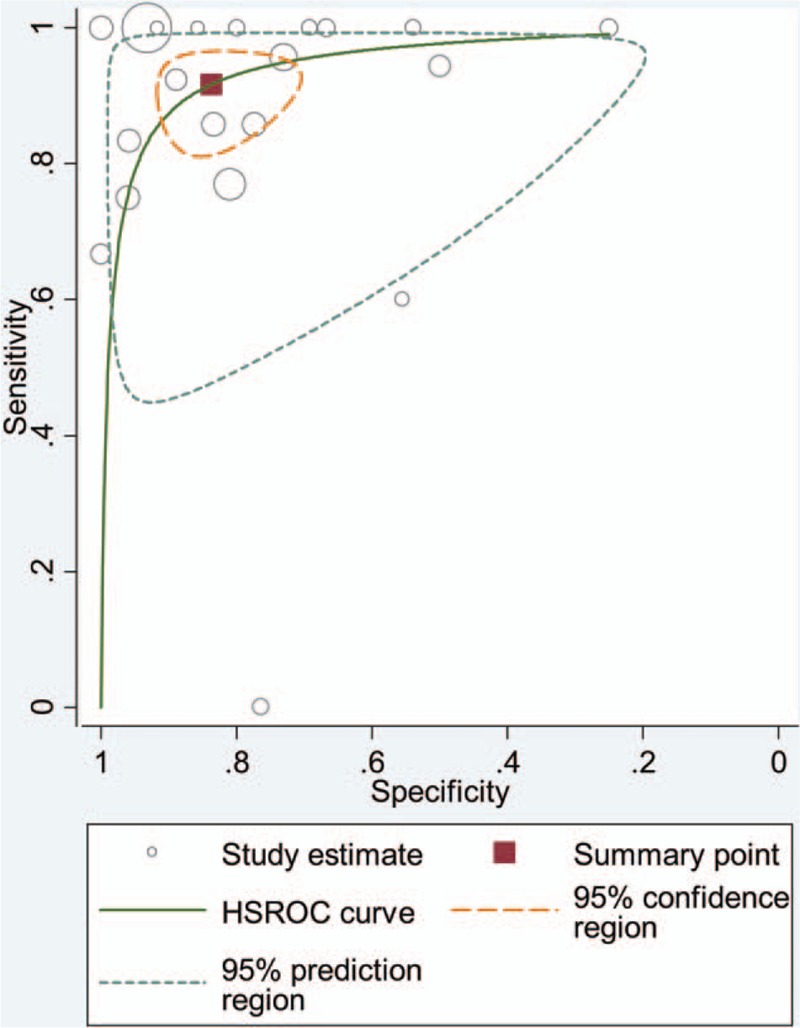
HSROC curve of SPECT for differentiating lymphoma from nonlymphoma FBLs in HIV patients. FBL = focal brain lesion, HIV = human immunodeficiency virus, HSROC = hierarchical summary receiver-operating characteristic, SPECT = single-photon emission computed tomography.
All 6 studies had a sensitivity of 100%. Regarding specificity, 4 out of 6 studies had a specificity of 100%, while the study by Pierce et al[22] reported a specificity of 91% and the study by Heald et al[18] reported a specificity of 75%. Further meta-analysis of PET studies could not be performed because no false negatives were reported for any of the 6 studies (ie, value of zero in 2 × 2 contingency table).
Three studies on MRS reported sensitivity of 0.71 (95% CI: 0.29–0.96), 0.50 (95% CI: 0.01–0.99), and 1.00 (95% CI: 0.63–1.00). The specificities were 0.83 (95% CI: 0.70–0.92), 0.60 (95% CI: 0.15–0.95), and 0.27 (95% CI: 0.08–0.55). Further meta-analysis could not be performed due to limited number of eligible studies.
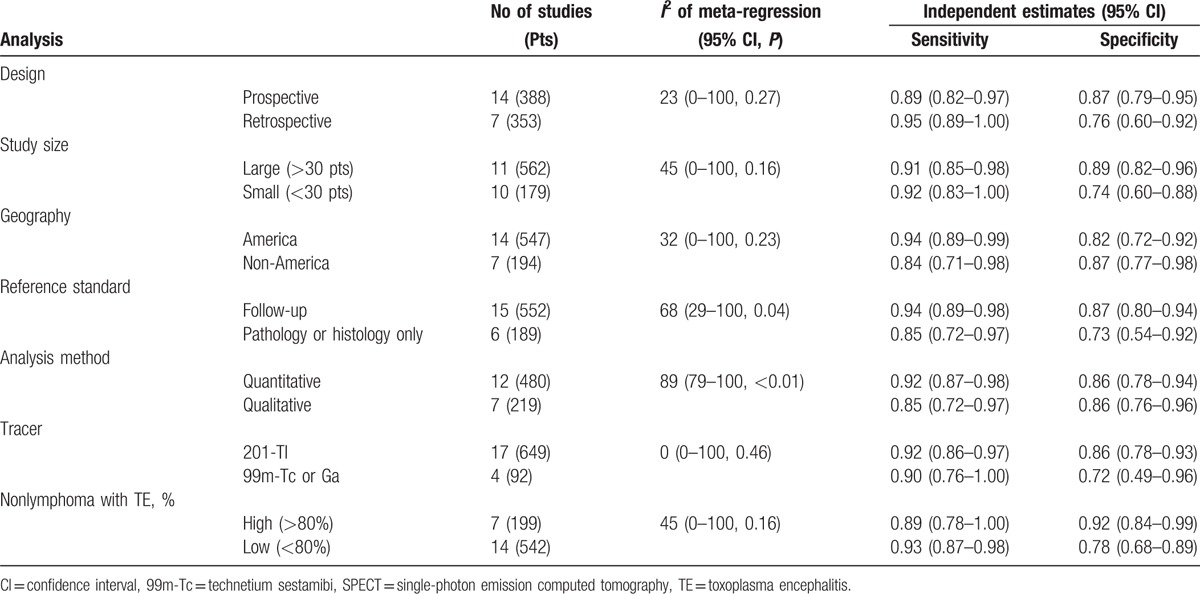
Bivariate meta-regression analysis was performed to detect the source of heterogeneity. We found that the analytical method used and reference standard contributed to the heterogeneity, while geography, study design, study size, tracer used, and proportion of TE in nonlymphoma cases did not contribute significantly to heterogeneity. The pooled sensitivity of studies using quantitative analysis was higher than that based on studies using visual inspection (0.94 vs 0.86). Both sensitivity (0.94 vs 0.85) and specificity (0.87 vs 0.73) in studies including follow-up as reference standard in addition to pathology and/or serology were higher than those that used only pathology and/or serology as the gold standard. The results of the subgroup meta-regression analysis for SPECT are summarized in Table 1.
Table 1.
Meta-regression analyses and diagnostic performance of SPECT in subgroups of study characteristics.
Sensitivity analysis using the leave-one-out approach demonstrated that the direction and magnitude of estimates were not influenced by any 1 study removed for SPECT (Supplemental Table 5). Deek funnel plot and regression test of asymmetry demonstrated a publication bias (P = 0.001) (Supplemental Fig. 1).
4. Discussion
Since the introduction of highly active antiretroviral therapy (HAART) in 1996, the epidemiology of CNS disease among HIV patients has been altered.[39] HAART reduces plasma viral load and thus significantly decreases the incidence of opportunistic infections such as toxoplasmosis and multifocal leukoencephalopathy in immunocompromised patients. However, the incidence of HIV-related lymphoma has been on the rise.[40] The exact pathogenic mechanism of PCNSL is not well understood, but the B-cell malignancies are consistently associated with Epstein–Barr virus. Lymphomagenesis typically occurs late in the course of HIV infection.[3]
In the post-HAART era, a correct early diagnosis of brain lymphoma is essential for HIV patients, since lymphoma is managed differently from toxoplasmosis and other opportunistic infections.[41] Unfortunately, brain biopsy is invasive while conventional imaging modalities have limited diagnostic accuracy.[6,12] Functional nuclear imaging modalities such as SPECT and PET have become increasingly popular diagnostic tools, as they provide images with high spatiotemporal resolution to evaluate perfusion or metabolism. SPECT relies on gamma ray emission from uptake of radiotracers such as Tc-99m, while PET utilizes positron-emitting radiotracers such as 18F-fluorodeoxyglucose.[42] MRS aims to query the biochemical composition of tissue by using the same 1H signals recognized by conventional MRI to determine relative concentrations of target brain metabolites such as N-acetylaspartate, choline, and creatine.[43] A generally accepted understanding of the use of SPECT, PET, and advanced MRI techniques to diagnose brain lymphoma is currently lacking due to the small number of published studies and limited cohort size. Thus, we performed a systematic review and meta-analysis to evaluate the diagnostic accuracy of SPECT, PET, and MRS in differentiating PCNSL from other FBLs in HIV patients.
In our meta-analysis, SPECT had a pooled sensitivity of 0.92 and a specificity of 0.84. We found moderate heterogeneity in both sensitivity and specificity meta-analysis for SPECT. Our meta-regression analysis demonstrated that the analytical method used contributed to the heterogeneity in sensitivity. Specifically, studies using a quantitative approach tended to have higher sensitivity than those using a qualitative approach. This may reflect the decreased subjectivity and higher precision associated with computer automation.[44] Another factor that contributed to the heterogeneity was the reference standard used. Most studies applied a combination of patient follow-up in addition to pathology and/or serology as their reference standard in determining the final diagnosis. We found that studies which used only pathology and/or serology as their gold standard had lower sensitivity and specificity than those which included patient follow-up as a reference standard in addition to pathology and/or serology. This suggests that the actual sensitivity and specificity of SPECT may be lower than expected. More studies using only pathology and/or serology as the gold standard are needed to reach a definite conclusion. Other factors that have been reported in the literature to further increase the sensitivity and/or specificity of SPECT include delayed imaging,[45] 99Tcm-sestamibi as tracer instead of 201TI,[15] larger lesion size,[46] and the addition of serum Toxoplasma IgG[47] and EBV PCR.[48] Giancola et al suggested that HAART may artificially increase 201TI uptake in patients with toxoplasmosis, resulting in lower diagnostic accuracy in the post-HAART era.[49] We could not compare the pooled sensitivity and specificity between pre- and post-HAART studies in the present study, since only 3 studies were published in the pre-HAART era.
PET may have higher sensitivity and specificity than SPECT. All 6 studies reported a sensitivity of 100%, while 4 out of 6 studies reported a specificity of 100%. However, the small number of studies and the lack of any false negatives in any of the 6 studies prevented meta-analysis.[50] Although PET allows rapid evaluation of the whole body, and can detect the primary lesion of brain metastasis, which can mimic PCNSL,[21] it has not been approved for this clinical indication due to limited supporting clinical data.[51] Additionally, PET is more expensive than SPECT and somewhat less accessible (especially worldwide).
Other imaging modalities such as CT and MRI have also been studied for their ability to differentiate PCNSL from other contrast-enhancing lesions in HIV patients. However, routine CT and MRI demonstrated only modest sensitivity and specificity.[5–10] In the present study, we included only 3 papers on MRS which contain extractable data, 2 of which reported only modest sensitivity and specificity in differentiating of lymphoma from other FBLs in HIV patients.[31,32,35] Other advanced MRI techniques, such as apparent diffusion coefficient (ADC) ratios, and regional cerebral blood volume (rCBV) have even less reported evidence.[34,52] According to our literature research, only 3 studies on diffusion[26,27,34] and 1 study on MR perfusion[28] were published on distinguishing lymphoma from other FBLs in HIV-infected patients. Two studies on diffusion demonstrated significant overlap in ADC ratios of toxoplasmosis and lymphoma.[26,27] The only study on MR perfusion reported both sensitivity and specificity of 100% in distinguishing lymphoma from other FBLs in 13 patients.[28] Further studies are need to investigate the diagnostic accuracy of these advanced MRI techniques.
We acknowledge several limitations of our study. First, some patients were under antitoxoplasmosis treatment when SPECT or PET was performed. Steroids and other drugs used can affect lymphoma presentations and complicate the differentiation of PCNSL from toxoplasmosis.[14] Second, the SPECT tracer 201-Tl and PET tracer 18F-fluorodeoxyglucose can accumulate in abscesses and inflammatory lesions, which can increase false-positive rates and interfere with diagnosis of dual pathology.[13] Third, small sample size, retrospective design, heterogeneous tumor characteristics, and different imaging parameters such as scanning time can contribute to bias in estimating diagnostic accuracy of SPECT, PET, and MRS. Fourth, lesion size was reported in a small number of studies, which precluded analysis of diagnostic accuracy based on a lesion size threshold. Finally, there was evidence of publication bias in our meta-analysis of SPECT, which suggests that inclusion of small studies may have skewed our estimates for test accuracy. However, such bias is not very concerning in meta-analysis of single outcome proportions because no effect estimate is calculated (ie, no comparison between groups is made).
5. Conclusion
SPECT has good diagnostic accuracy in differentiating PCNSL from other FBLs in HIV patients, but the actual sensitivity and specificity may be lower than expected when only pathology and/or serology was used as the gold standard. PET may be superior but has less supporting clinical data and is more expensive.
Supplementary Material
Supplementary Material
Acknowledgments
This work was supported by the Natural Science Foundation of China (81301988 to L.Y.), and China Ministry of Education Doctoral Program Spot Foundation (20130162120061 to L.Y.). Shenghua Yuying Project of Central South University to L.Y.
Footnotes
Abbreviations: FBL = focal brain lesion, HAART= highly active antiretroviral therapy, PCNSL = primary central nervous system lymphoma.
MY and JS contributed equally this work and considered cofirst authors.
Authorship: MY and HXB, study conception and writing for the primary draft; JS, YT, and XT, data analysis and critical revision of the manuscript; LJS, ZZ, and JZ, access to data and revision of the draft for important intellectual content; MDF, PZ, and BX, review of the study and data interpretation; and LY, data acquisition and final editing.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol 2005;4:543–55. [DOI] [PubMed] [Google Scholar]
- [2].Tan IL, Smith BR, von Geldern G, et al. HIV-associated opportunistic infections of the CNS. Lancet Neurol 2012;11:605–17. [DOI] [PubMed] [Google Scholar]
- [3].Flinn IW, Ambinder RF. AIDS primary central nervous system lymphoma. Curr Opin Oncol 1996;8:373–6. [DOI] [PubMed] [Google Scholar]
- [4].Baumgartner JE, Rachlin JR, Beckstead JH, et al. Primary central nervous system lymphomas: natural history and response to radiation therapy in 55 patients with acquired immunodeficiency syndrome. J Neurosurg 1990;73:206–11. [DOI] [PubMed] [Google Scholar]
- [5].Shyam babu C, Satishchandra P, Mahadevan A, et al. Usefulness of stereotactic biopsy and neuroimaging in management of HIV-1 Clade C associated focal brain lesions with special focus on cerebral toxoplasmosis. Clin Neurol Neurosurg 2013;115:995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ciricillo SF, Rosenblum ML. Use of CT and MR imaging to distinguish intracranial lesions and to define the need for biopsy in AIDS patients. J Neurosurg 1990;73:720–4. [DOI] [PubMed] [Google Scholar]
- [7].Dina TS. Primary central nervous system lymphoma versus toxoplasmosis in AIDS. Radiology 1991;179:823–8. [DOI] [PubMed] [Google Scholar]
- [8].Gill PS, Graham RA, Boswell W, et al. A comparison of imaging, clinical, and pathologic aspects of space-occupying lesions within the brain in patients with acquired immune deficiency syndrome. Am J Physiol Imaging 1986;1:134–41. [PubMed] [Google Scholar]
- [9].Goldstein JD, Zeifer B, Chao C, et al. CT appearance of primary CNS lymphoma in patients with acquired immunodeficiency syndrome. J Comput Assist Tomogr 1991;15:39–44. [DOI] [PubMed] [Google Scholar]
- [10].Miller RF, Hall-Craggs MA, Costa DC, et al. Magnetic resonance imaging, thallium-201 SPET scanning, and laboratory analyses for discrimination of cerebral lymphoma and toxoplasmosis in AIDS. Sex Transm Infect 1998;74:258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Castagna A, Cinque P, d’Amico A, et al. Evaluation of contrast-enhancing brain lesions in AIDS patients by means of Epstein-Barr virus detection in cerebrospinal fluid and 201thallium single photon emission tomography. AIDS 1997;11:1522–3. [PubMed] [Google Scholar]
- [12].Lee AM, Bai HX, Zou Y, et al. Safety and diagnostic value of brain biopsy in HIV patients: a case series and meta-analysis of 1209 patients. J Neurol Neurosurg Psychiatry 2016;87:722–33. [DOI] [PubMed] [Google Scholar]
- [13].Antinori A, De Rossi G, Ammassari A, et al. Value of combined approach with thallium-201 single-photon emission computed tomography and Epstein-Barr virus DNA polymerase chain reaction in CSF for the diagnosis of AIDS-related primary CNS lymphoma. J Clin Oncol 1999;17:554–60. [DOI] [PubMed] [Google Scholar]
- [14].Lorberboym M, Estok L, Machac J, et al. Rapid differential diagnosis of cerebral toxoplasmosis and primary central nervous system lymphoma by thallium-201 SPECT. J Nucl Med 1996;37:1150–4. [PubMed] [Google Scholar]
- [15].Naddaf SY, Akisik MF, Aziz M, et al. Comparison between 201Tl-chloride and 99Tc(m)-sestamibi SPET brain imaging for differentiating intracranial lymphoma from non-malignant lesions in AIDS patients. Nucl Med Commun 1998;19:47–53. [DOI] [PubMed] [Google Scholar]
- [16].O’Malley JP, Ziessman HA, Kumar PN, et al. Diagnosis of intracranial lymphoma in patients with AIDS: value of 201TI single-photon emission computed tomography. AJR Am J Roentgenol 1994;163:417–21. [DOI] [PubMed] [Google Scholar]
- [17].Ruiz A, Ganz WI, Post MJ, et al. Use of thallium-201 brain SPECT to differentiate cerebral lymphoma from toxoplasma encephalitis in AIDS patients. AJNR Am J Neuroradiol 1994;15:1885–94. [PMC free article] [PubMed] [Google Scholar]
- [18].Heald AE, Hoffman JM, Bartlett JA, et al. Differentiation of central nervous system lesions in AIDS patients using positron emission tomography (PET). Int J STD AIDS 1996;7:337–46. [DOI] [PubMed] [Google Scholar]
- [19].Hoffman JM, Waskin HA, Schifter T, et al. FDG-PET in differentiating lymphoma from nonmalignant central nervous system lesions in patients with AIDS. J Nucl Med 1993;34:567–75. [PubMed] [Google Scholar]
- [20].Lewitschnig S, Gedela K, Toby M, et al. (1)(8)F-FDG PET/CT in HIV-related central nervous system pathology. Eur J Nucl Med Mol Imaging 2013;40:1420–7. [DOI] [PubMed] [Google Scholar]
- [21].O’Doherty MJ, Barrington SF, Campbell M, et al. PET scanning and the human immunodeficiency virus-positive patient. J Nucl Med 1997;38:1575–83. [PubMed] [Google Scholar]
- [22].Pierce MA, Johnson MD, Maciunas RJ, et al. Evaluating contrast-enhancing brain lesions in patients with AIDS by using positron emission tomography. Ann Intern Med 1995;123:594–8. [DOI] [PubMed] [Google Scholar]
- [23].Villringer K, Jager H, Dichgans M, et al. Differential diagnosis of CNS lesions in AIDS patients by FDG-PET. J Comput Assist Tomogr 1995;19:532–6. [DOI] [PubMed] [Google Scholar]
- [24].D’Amico A, Messa C, Castagna A, et al. Diagnostic accuracy and predictive value of 201T1 SPET for the differential diagnosis of cerebral lesions in AIDS patients. Nucl Med Commun 1997;18:741–50. [DOI] [PubMed] [Google Scholar]
- [25].Licho R, Litofsky NS, Senitko M, et al. Inaccuracy of Tl-201 brain SPECT in distinguishing cerebral infections from lymphoma in patients with AIDS. Clin Nucl Med 2002;27:81–6. [DOI] [PubMed] [Google Scholar]
- [26].Camacho DL, Smith JK, Castillo M. Differentiation of toxoplasmosis and lymphoma in AIDS patients by using apparent diffusion coefficients. AJNR Am J Neuroradiol 2003;24:633–7. [PMC free article] [PubMed] [Google Scholar]
- [27].Schroeder PC, Post MJ, Oschatz E, et al. Analysis of the utility of diffusion-weighted MRI and apparent diffusion coefficient values in distinguishing central nervous system toxoplasmosis from lymphoma. Neuroradiology 2006;48:715–20. [DOI] [PubMed] [Google Scholar]
- [28].Ernst TM, Chang L, Witt MD, et al. Cerebral toxoplasmosis and lymphoma in AIDS: perfusion MR imaging experience in 13 patients. Radiology 1998;208:663–9. [DOI] [PubMed] [Google Scholar]
- [29].Chong WK, Sweeney B, Wilkinson ID, et al. Proton spectroscopy of the brain in HIV infection: correlation with clinical, immunologic, and MR imaging findings. Radiology 1993;188:119–24. [DOI] [PubMed] [Google Scholar]
- [30].Jarvik JG, Lenkinski RE, Grossman RI, et al. Proton MR spectroscopy of HIV-infectedpatients: characterization of abnormalities with imaging and clinical correlation. Radiology 1993;186:739–44. [DOI] [PubMed] [Google Scholar]
- [31].Chang L, Miller BL, McBride D, et al. Brain lesions in patients with AIDS: H-1 MR spectroscopy. Radiology 1995;197:525–31. [DOI] [PubMed] [Google Scholar]
- [32].Chinn RJ, Wilkinson ID, Hall-Craggs MA, et al. Toxoplasmosis and primary central nervous system lymphoma in HIV infection: diagnosis with MR spectroscopy. Radiology 1995;197:649–54. [DOI] [PubMed] [Google Scholar]
- [33].Pomper MG, Constantinides CD, Barker PB, et al. Quantitative MR spectroscopic imaging of brain lesions in patients with AIDS: correlation with [11C-methyl]thymidine PET and thallium-201 SPECT. Acad Radiol 2002;9:398–409. [DOI] [PubMed] [Google Scholar]
- [34].Zacharia TT, Law M, Naidich TP, et al. Central nervous system lymphoma characterization by diffusion-weighted imaging and MR spectroscopy. J Neuroimaging 2008;18:411–7. [DOI] [PubMed] [Google Scholar]
- [35].Simone IL, Federico F, Tortorella C, et al. Localised 1H-MR spectroscopy for metabolic characterisation of diffuse and focal brain lesions in patients infected with HIV. J Neurol Neurosurg Psychiatry 1998;64:516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Westwood TD, Hogan C, Julyan PJ, et al. Utility of FDG-PETCT and magnetic resonance spectroscopy in differentiating between cerebral lymphoma and non-malignant CNS lesions in HIV-infected patients 2013. Eur J Radiol 2013;82:e374–9. [DOI] [PubMed] [Google Scholar]
- [38].DeLaPena RC, Ketonen L, Villanueva-Meyer J. Imaging of brain tumors in AIDS patients by means of dual-isotope thallium-201 and technetium-99m sestamibi single-photon emission tomography. Eur J Nucl Med 1998;25:1404–11. [DOI] [PubMed] [Google Scholar]
- [39].Sacktor N, Lyles RH, Skolasky R, et al. HIV-associated neurologic disease incidence changes: Multicenter AIDS Cohort Study, 1990–1998. Neurology 2001;56:257–60. [DOI] [PubMed] [Google Scholar]
- [40].Schabet M. Epidemiology of primary CNS lymphoma. J Neurooncol 1999;43:199–201. [DOI] [PubMed] [Google Scholar]
- [41].Ekenel M, Deangelis LM. Treatment of primary central nervous system lymphoma. Curr Treat Options Neurol 2007;9:271–82. [DOI] [PubMed] [Google Scholar]
- [42].Lammertsma AA. PET/SPECT: functional imaging beyond flow. Vision Res 2001;41:1277–81. [DOI] [PubMed] [Google Scholar]
- [43].Preul MC, Caramanos Z, Collins DL, et al. Accurate, noninvasive diagnosis of human brain tumors by using proton magnetic resonance spectroscopy. Nat Med 1996;2:323–5. [DOI] [PubMed] [Google Scholar]
- [44].Kessler LS, Ruiz A, Donovan Post MJ, et al. Thallium-201 brain SPECT of lymphoma in AIDS patients: pitfalls and technique optimization. AJNR Am J Neuroradiol 1998;19:1105–9. [PMC free article] [PubMed] [Google Scholar]
- [45].Lorberboym M, Wallach F, Estok L, et al. Thallium-201 retention in focal intracranial lesions for differential diagnosis of primary lymphoma and nonmalignant lesions in AIDS patients. J Nucl Med 1998;39:1366–9. [PubMed] [Google Scholar]
- [46].Young RJ, Ghesani MV, Kagetsu NJ, et al. Lesion size determines accuracy of thallium-201 brain single-photon emission tomography in differentiating between intracranial malignancy and infection in AIDS patients. AJNR Am J Neuroradiol 2005;26:1973–9. [PMC free article] [PubMed] [Google Scholar]
- [47].Skiest DJ, Erdman W, Chang WE, et al. SPECT thallium-201 combined with Toxoplasma serology for the presumptive diagnosis of focal central nervous system mass lesions in patients with AIDS. J Infect 2000;40:274–81. [DOI] [PubMed] [Google Scholar]
- [48].Hussain FS, Hussain NS. Clinical utility of Thallium-201 single photon emission computed tomography and cerebrospinal fluid Epstein-Barr virus detection using polymerase chain reaction in the diagnosis of AIDS-related primary central nervous system lymphoma. Cureus 2016;8:e606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Giancola ML, Rizzi EB, Schiavo R, et al. Reduced value of thallium-201 single-photon emission computed tomography in the management of HIV-related focal brain lesions in the era of highly active antiretroviral therapy. AIDS Res Hum Retroviruses 2004;20:584–8. [DOI] [PubMed] [Google Scholar]
- [50].Takwoingi Y, Guo B, Riley RD, et al. Performance of methods for meta-analysis of diagnostic test accuracy with few studies or sparse data. Stat Methods Med Res 2015;[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Berger M, Gould MK, Barnett PG. The cost of positron emission tomography in six United States Veterans Affairs hospitals and two academic medical centers. AJR Am J Roentgenol 2003;181:359–65. [DOI] [PubMed] [Google Scholar]
- [52].Laissy JP, Soyer P, TebbouneJ, et al. Contrast-enhanced fast MRI in differentiating brain toxoplasmosis and lymphoma in AIDS patients. J Comput Assist Tomogr 1994;18:714–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


