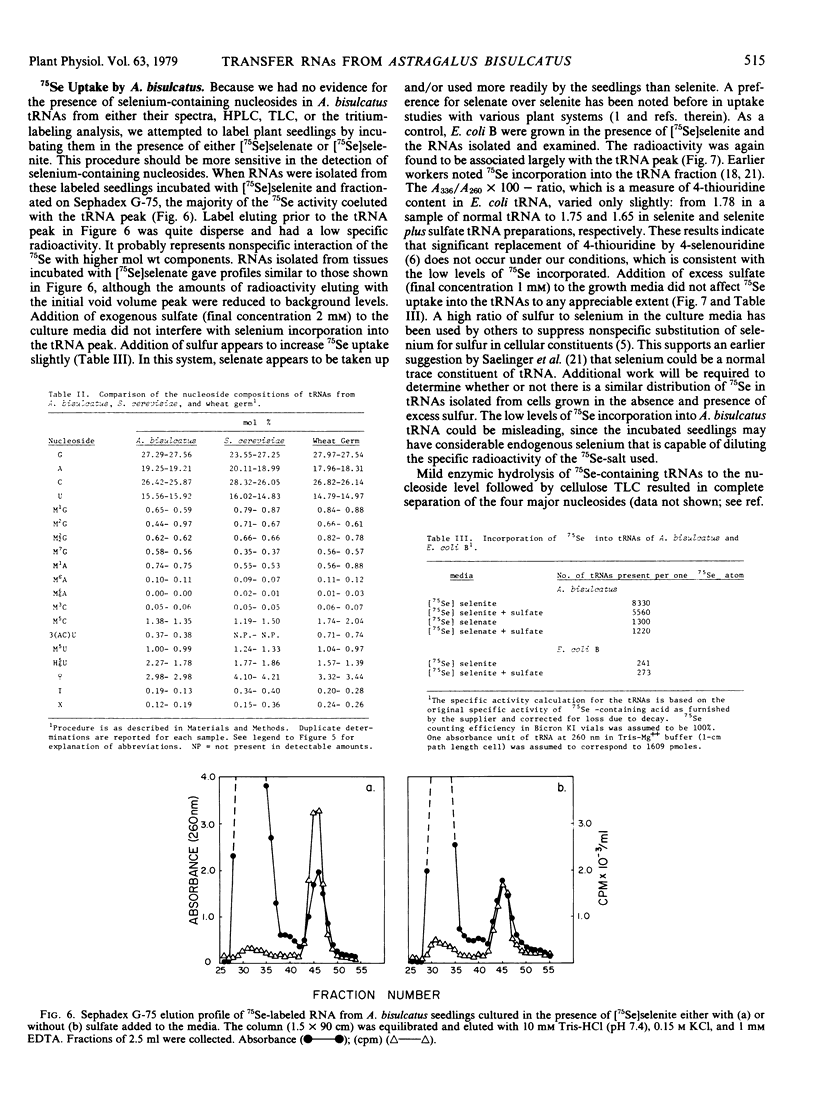
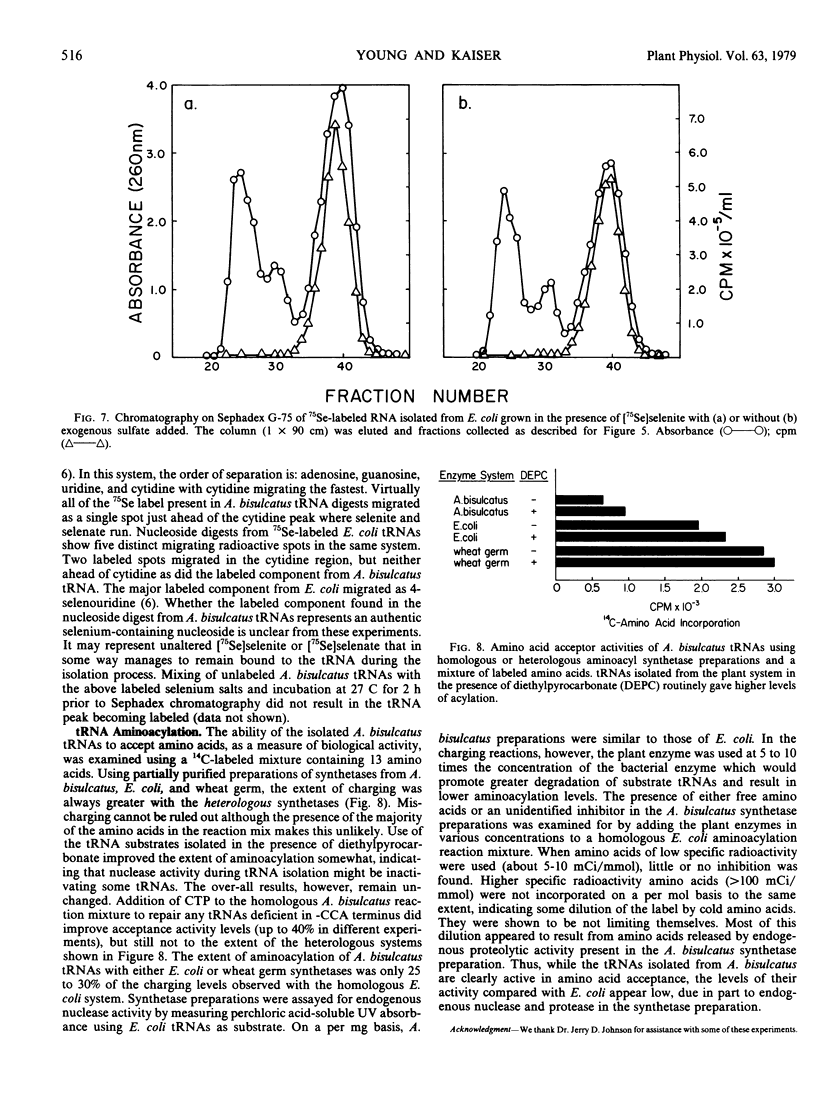
Abstract
A procedure has been developed for the isolation of transfer RNA from the selenium accumulator plant Astragalus bisulcatus. This material appears free of interfering phenolic compounds, has a high guanosine to cytidine ratio, shows a major and modified nucleoside composition characteristic of plant transfer RNAs, and exhibits chromatographic and electrophoretic properties similar to transfer RNAs from other well studied bacterial and plant systems. RNAs isolated from A. bisulcatus seedlings incubated in the presence of 75Se indicate some incorporation of radioactivity into the transfer RNAs, but at extremely low levels. The transfer RNAs were active in accepting amino acids, although their over-all levels of activity appeared low when compared with those from a homologous Escherichia coli aminoacylation reaction system.
Full text
PDF
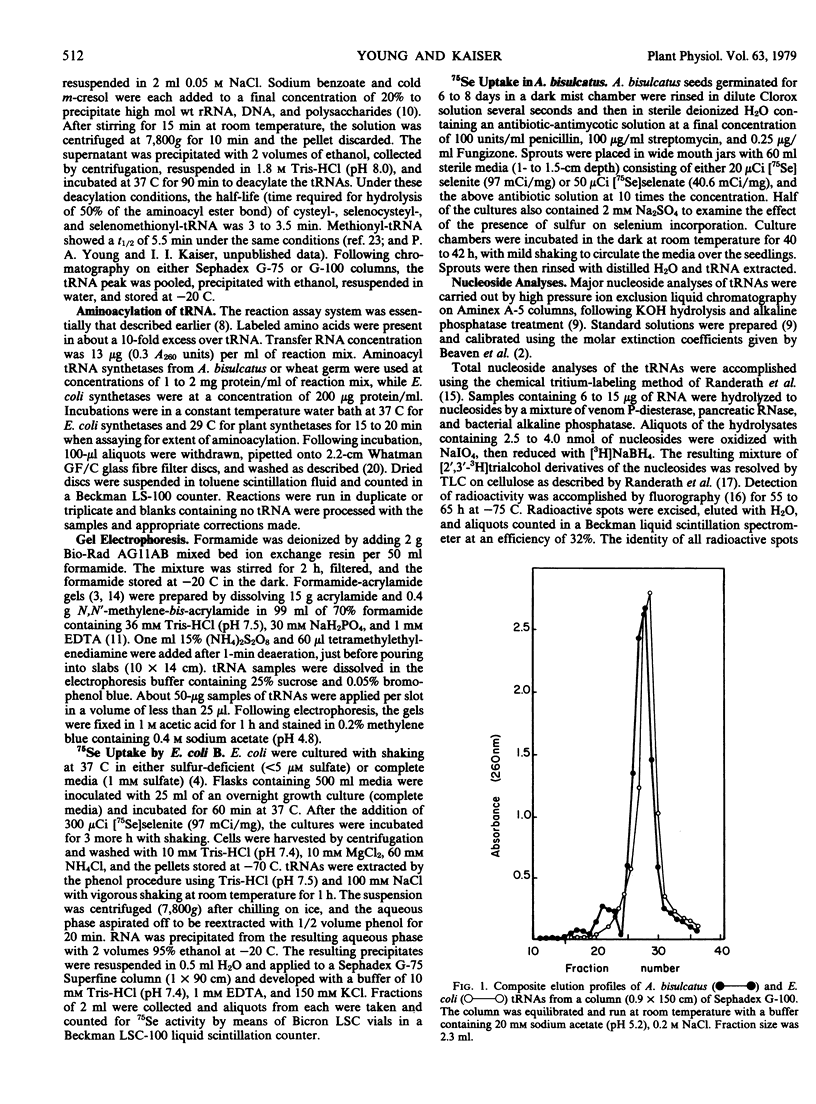
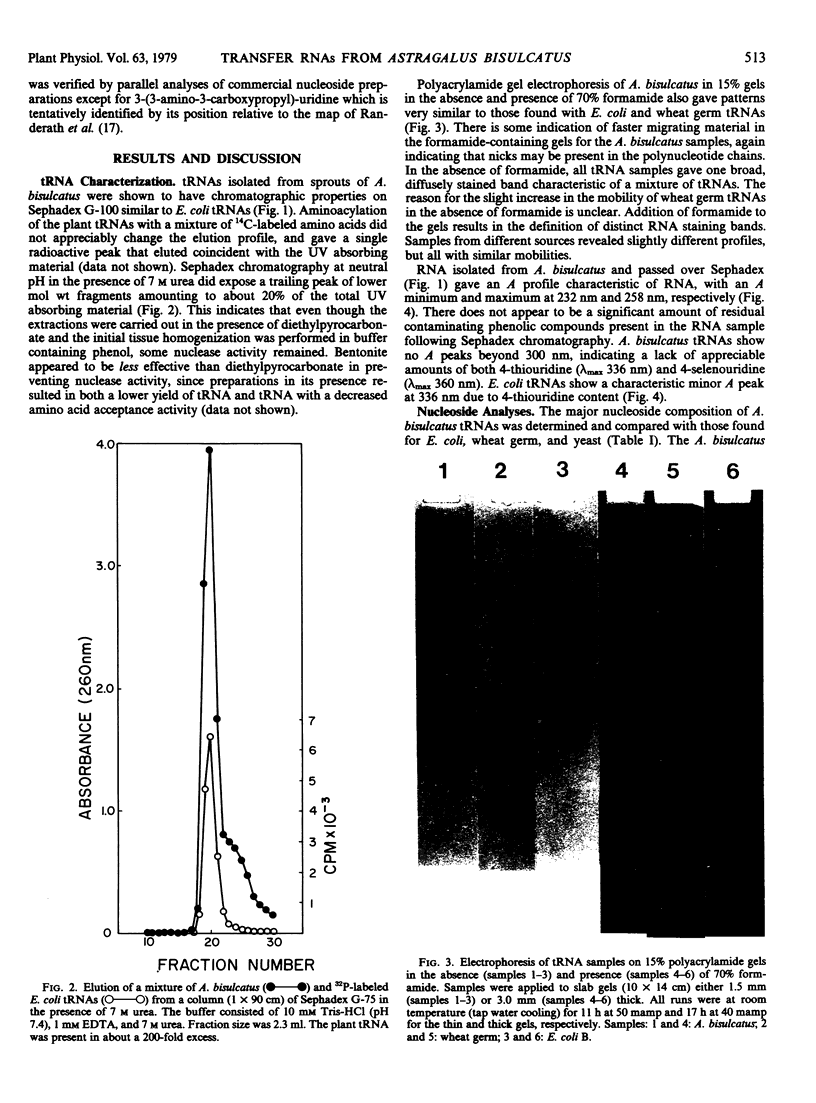
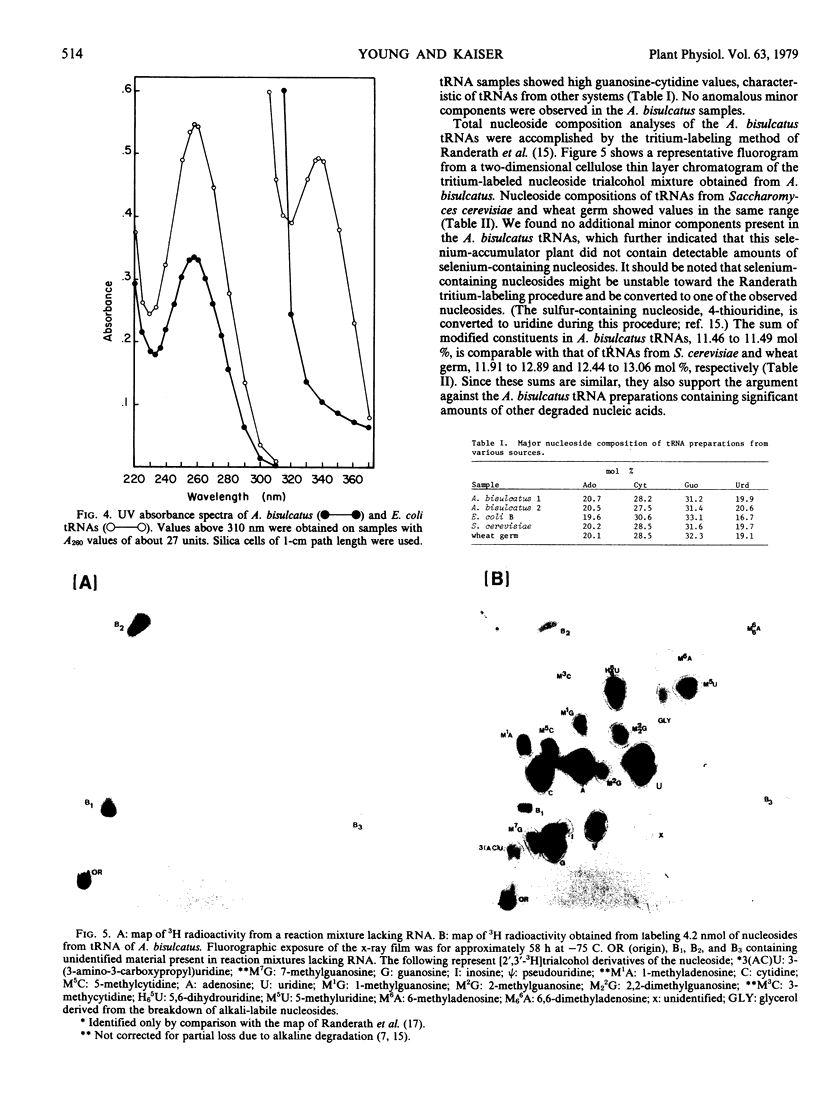

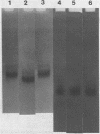
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boedtker H., Frischauf A. M., Lehrach H. Isolation and translation of calvaria procollagen messenger ribonucleic acids. Biochemistry. 1976 Nov 2;15(22):4765–4770. doi: 10.1021/bi00667a003. [DOI] [PubMed] [Google Scholar]
- Carbon J. A., Hung L., Jones D. S. A reversible oxidative in activation of specific transfer RNA species. Proc Natl Acad Sci U S A. 1965 May;53(5):979–986. doi: 10.1073/pnas.53.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone J. E., Del Río R. M., Davis J. N., Stadtman T. C. Chemical characterization of the selenoprotein component of clostridial glycine reductase: identification of selenocysteine as the organoselenium moiety. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2659–2663. doi: 10.1073/pnas.73.8.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J. L., McConnell K. P. The presence of 4-selenouridine in Escherichia coli tRNA. Biochim Biophys Acta. 1974 Sep 27;366(1):109–113. doi: 10.1016/0005-2787(74)90323-2. [DOI] [PubMed] [Google Scholar]
- Johnson J. D., Horowitz J. Characterization of ribosomes and RNAs from Mycoplasma hominis. Biochim Biophys Acta. 1971 Oct 14;247(2):262–279. doi: 10.1016/0005-2787(71)90675-7. [DOI] [PubMed] [Google Scholar]
- Kaiser I. I., Young P. A. Determination of 5-fluorouridine and 5-fluorocytidine in RNA hydrolysates using high-performance ion-exclusion chromatography. J Chromatogr. 1975 Aug 20;111(1):242–245. doi: 10.1016/s0021-9673(01)80172-2. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinder J. C., Staynov D. Z., Gratzer W. B. Electrophoresis of RNA in formamide. Biochemistry. 1974 Dec 17;13(26):5373–5378. doi: 10.1021/bi00723a019. [DOI] [PubMed] [Google Scholar]
- Prasada Rao Y. S., Cherayil J. D. Number and proportion of selenonucleosides in the transfer RNA of Escherichia coli. Life Sci. 1974 May 16;14(10):2051–2059. doi: 10.1016/0024-3205(74)90423-8. [DOI] [PubMed] [Google Scholar]
- Randerath E., Yu C. T., Randerath K. Base analysis of ribopolynucleotides by chemical tritium labeling: a methodological study with model nucleosides and purified tRNA species. Anal Biochem. 1972 Jul;48(1):172–198. doi: 10.1016/0003-2697(72)90181-9. [DOI] [PubMed] [Google Scholar]
- Randerath K. An evaluation of film detection methods for weak beta-emitters, particularly tritium. Anal Biochem. 1970 Mar;34:188–205. doi: 10.1016/0003-2697(70)90100-4. [DOI] [PubMed] [Google Scholar]
- Randerath K., Randerath E., Chia L. S., Nowak B. J. Base analysis of ribopolynucleotides by chemical tritium labeling: an improved mapping procedure for nucleoside trialcohols. Anal Biochem. 1974 May;59(1):263–271. doi: 10.1016/0003-2697(74)90032-3. [DOI] [PubMed] [Google Scholar]
- Rubin I. B., Kelmers A. D., Goldstein G. The determination of transfer ribonucleic acid by aminoacylation. I. Leucine and phenylalanine transfer ribonucleic acid from E. coli B. Anal Biochem. 1967 Sep;20(3):533–544. doi: 10.1016/0003-2697(67)90298-9. [DOI] [PubMed] [Google Scholar]
- Saelinger D. A., Hoffman J. L., McConnell K. P. Biosynthesis of selenobases in transfer RNA by Escherichia coli. J Mol Biol. 1972 Aug 14;69(1):9–17. doi: 10.1016/0022-2836(72)90020-4. [DOI] [PubMed] [Google Scholar]
- Stadtman T. C. Selenium biochemistry. Science. 1974 Mar 8;183(4128):915–922. doi: 10.1126/science.183.4128.915. [DOI] [PubMed] [Google Scholar]
- Young P. A., Kaiser I. I. Aminoacylation of Escherichia coli cysteine tRNA by selenocysteine. Arch Biochem Biophys. 1975 Dec;171(2):483–489. doi: 10.1016/0003-9861(75)90057-0. [DOI] [PubMed] [Google Scholar]