Abstract
Triosephosphate isomerase (TPI) is highly expressed in many human cancers and is involved in migration and invasion of cancer cells. However, TPI clinicopathological significance and prognostic value in gastric cancer (GC) are not yet well defined. The aim of the present work was to evaluate TPI expression in GC tissue and its prognostic value in GC patients.
TPI expression was analyzed in 92 primary GC tissues and 80 adjacent normal mucosa tissues from GC patients undergoing gastrectomy by immunohistochemical analysis of tissue microarrays (TMAs). Univariate and multivariate analyses were performed to investigate TPI prognostic significance in GC patients.
Immunohistochemical staining score showed that TPI expression in cancer tissues was significantly higher than in adjacent normal mucosa (P < .001). Univariate analysis revealed that TPI expression, depth of invasion, lympho node metastasis, tumor node metastasis (TNM) stage, and tumor diameter were associated with negative prognostic predictors for overall survival in GC patients (P < .05). High TPI expression represented a significant predictor of shorter survival in GC patients with positive lymphatic metastasis (P = .022) and tumor diameter >5 cm (P = .018). Cox multivariate analysis identified TPI expression, TNM stage, and tumor diameter as independent prognostic factors in GC patients.
TPI expression might be considered as a novel prognostic factor to evaluate GC patients’ survival.
Keywords: gastric cancer, survival, triosephosphate isomerase
1. Introduction
Gastric cancer (GC) is one of the most malignant gastrointestinal neoplasms worldwide.[1] It ranks 1st among all gastrointestinal tumors in some Asian countries such as China and Japan.[2] In addition, postoperative patients, especially those with high GC stage, show a poor long-term survival.[3] Several factors including HIF-1, P53, and HER-2 are related with GC, and some of them are associated with GC patients’ prognosis.[3,4] However, these factors trend in GC is not enough to clarify GC complex pathogenesis; thus, key markers involved in GC development, progression, and prognosis need to be further identified.
Triosephosphate isomerase (TPI), also called HEL-S-49, TIM, TPI1, or TPID, is a gene encoding the glycolytic enzyme triosephosphate which maps to 12p13.[5] TPI enzyme is necessary for cell growth and maintenance.[6] Its main function is to catalyze the interconversion of dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (G3P) in glycolysis pathway and in other metabolic pathway.[7] Several studies have reported that TPI was over-expressed in many cancers, such as lung adenocarcinoma, bladder squamous cell carcinoma, and breast carcinoma.[8–10] TPI is nowadays considered involved in tumor development through promoting tumor cells proliferation, migration, and invasion. Wang et al[11] reported that TPI was markedly increased in the highly metastatic GBC-SD18H cell line (from gallbladder carcinoma GBC-SD cell line) compared to the poorly metastatic GBC-SD18L cell line. Besides, TPI was found with an elevated expression in node-positive breast carcinomas compared to node-negative breast carcinomas.[12] Linge et al[13] reported that TPI expression in uveal melanoma tissue of patients who had subsequently developed metastatic disease was higher than those who had not. Thus, they silenced TPI in 92.1 uveal melanoma cells and found that their invasion and motility ability were decreased. These studies indicated that TPI may be associated with tumor progression and prognosis of patients to some extent.
However, the expression of TPI in GC tissue and its correlation with GC have not been reported up to now. This study aimed to determine TPI expression in GC and to explore its clinicopathological characteristics and prognostic value in GC patients in order to evaluate TPI potential role as a marker in GC pathogenesis.
2. Materials and methods
2.1. Patients and tissue samples
Paraffin-embedded tissue samples were obtained from National Engineering Center for Biochip at Shanghai (Shanghai Outdo Biotech Co. Ltd., Shanghai, China). All the patients were diagnosed with GC by histology between 2006 and 2007. These tissue samples included 92 cancer tissues and corresponding adjacent normal mucosa. Patients’ characteristics and clinicopathological data included age, gender, tumor stage, pathological type, lymph node metastases, and tumor diameter. Patients’ tumor stages were assessed following the Seven Edition of American Joint Committee on Cancer.
Patients were monitored from the end of the surgery until September 2014 or their death. During this period, patients’ events died because of GC were defined as complete data, otherwise they were defined as censored data. Lost to follow-up or death for other reasons belonged to censored data. Censored data were defined as the interval between surgery to the last follow-up time or to the time of death for other reasons. The inter quartile range (IQR) of the follow-up time was 72 months (range, from 3 to 90 months). Among all patients, 10 were lost to follow up until the end of the study. However, the lose track patients had been lived for 5 years before lost to follow-up. Twenty patients were still alive in September 2014. Sixty two patients died during the follow-up period.
All procedures performed in this study involving human participants were in accordance with the ethical standards of the Ethics Committee of Affiliated Hospital of Guangdong Medical University and with the Ethical Principles for Medical Research Involving Human Subjects outlined in the Helsinki Declaration in 1975 (revised in 2000). Any systematic data gathering effort in patients were approved by an Institutional Review Board (IRB).
2.2. Tissue microarray and immunohistochemistry
Tissue microarray (TMA) was constructed as previously described.[14] TMA contained 92 GC patients and corresponding adjacent normal mucosa. Originally, tumor to control ratio was 1:1. However, 12 adjacent normal mucosa samples were damaged by accident during chip preparation. Thus, the control samples were 80.
Immunohistochemical assay was performed on TMA chips. The chip was deparaffinized in xylene twice and then rehydrated in ethanol gradients. A 3% H2O2 solution was used to block endogenous peroxidase, then the chip was washed in phosphate-buffered saline (PBS) for 3 times and immersed in 10 mM citrate buffer (pH 6.0) in boiling water twice for 5 minutes for antigen retrieval. The chip was washed 3 times with PBS and incubated with goat anti-TPI antibody (Abcam) at 1:100 dilution at 4 °C overnight (12–14 hours). The next day, the chip was washed 3 times with PBS and incubated with secondary antibody for 30 minutes, washed 3 times with PBS, and incubated with horseradish peroxidase-labeled streptavidin for 30 minutes. The chip was incubated for 3 minutes with diaminobenzidine (DAB) after PBS washing, counterstained with hematoxylin, dehydrated, immersed in xylene, and permanently mounted with resinous mounting medium.
The staining was analyzed independently by 2 pathologists using the same judgement criteria. The staining score was evaluated based on criteria previously described. The mean scores value of the 2 observers was adapted for further analysis to avoid the interobserver variability. According to the cancer cell staining intensity, TPI staining results were classified as follows: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining. Positive cancer cells were classified as follows: score 0, no tumor cells showing positive staining; score 1, 1% to 24% positive cells; score 2, 25% to 49% positive cells; score 3, 50% to 74% positive cells; and score 4, 75% to 100% positive cells.[15] The percentage of positive cells and the staining intensity were added as the final score. TPI score 0 to 3 in tissues was considered low expression; score 4 to 6 was considered high expression.[13,16]
2.3. Statistical analysis
Statistical analysis was carried out using SPSS15.0 software (Chicago, IL). The period between the surgery date and the date of death or last follow-up was defined as overall survival (OS). Chi-square test and Fisher exact test were conducted to measure the difference between TPI expression and patients’ characteristics or clinicopathological factors. Kaplan–Meier analysis and log-rank test were used for survival analysis. Proportional hazards (PH) assumption was used to evaluate whether the variables were suitable for the conditions of multivariable Cox PH models.[15,17] Then, we conducted multivariable Cox PH models (forward likelihood ratio model) to screen independent prognostic factors when conditions were applicable. The limiting condition to enter the model was when 0.05 < α < 0.1. Prognostic index (PI) score was calculated by the following formula: PI = β1 x1 + β2 x2 + β3 x3, where β were regression coefficients in multivariate Cox regression analysis.[18]P value (2 sides) < .05 were considered statistically significant.
3. Results
3.1. TPI expression in GC
TPI expression was evaluated by immunohistochemical using TMA in 92 GC patients’ tissues and 80 para-carcinoma normal tissues. TPI positive staining was observed in the cytoplasm and/or in the cytoblast. The results showed that TPI expression in GC tissues was markedly higher than in para-carcinoma tissues (P < .001). Cancer tissue staining results showed that 13/92 specimens (14.13%) were characterized by low-intensity staining (0–3), and 79/92 specimens (85.87%) were highly stained (≥4), while adjacent tissue results showed that 47/80 specimens (58.75%) were characterized by low-intensity staining and 33/80 specimens (41.25%) were highly stained (Table 1; Fig. 1A–F).
Table 1.
Expression of TPI in GC tissue and para-carcinoma tissue by immunohistochemistry, n (%).
Figure 1.
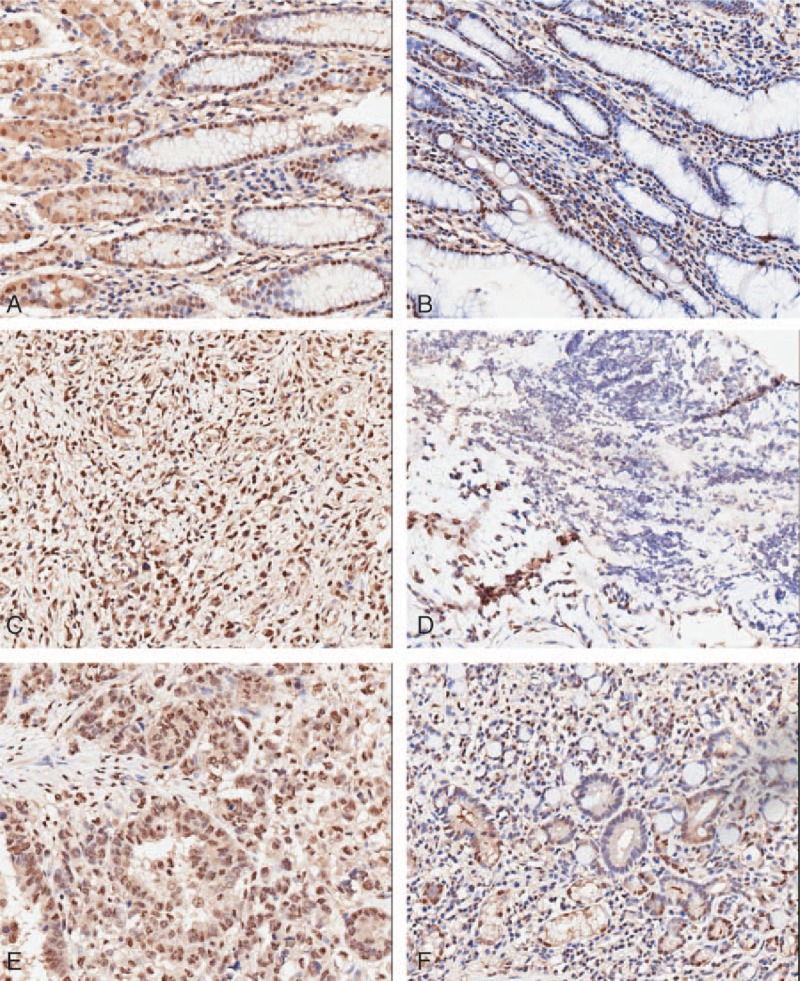
TPI expression in GC tissue and in para-carcinoma tissue by immunostaining of TMA sections. (A) TPI high-intensity staining in para-carcinoma tissue. (B) TPI low-intensity staining in para-carcinoma tissue. (C, E) TPI high-intensity staining in GC specimens. (D, F) TPI low-intensity staining in GC specimens (×100 magnification). GC = gastric cancer, TMA = tissue microarray, TPI = triosephosphate isomerase.
3.2. Correlation between TPI expression and clinicopathological parameters in GC patients
As shown in Table 2, 58 patients were male and 34 patients were female. The histological type of cancer of the 92 patients was adenocarcinoma. TPI expression in tumor tissue was significantly associated only with gender (P = .002). Thirty four female tissues were characterized by TPI high expression (100%), and 47/58 male specimens (81%) were characterized by TPI high expression. We did not found any significant association between TPI expression and other patients’ characteristics or clinicopathological characteristics (age, Borrmann type, depth of invasion, lymph node metastasis, TMN stage, and tumor diameter).
Table 2.
Correlation between TPI expression and GC patients’ characteristics or clinicopathologic characteristics (n = 92).

3.3. Survival analysis
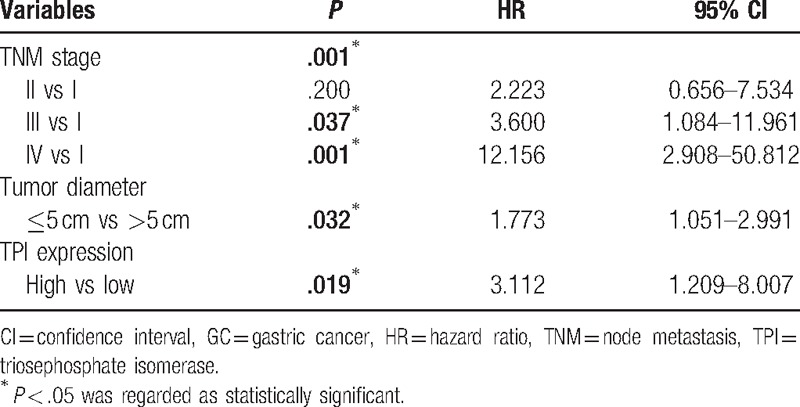
Our present study showed that the median OS of all GC patients was 49 months. Thus, we analyzed whether TPI expression in GC affected the clinical prognosis. Univariate analysis showed that depth of invasion (P = .002), lymph node metastasis (P = .001), tumor node metastasis (TNM) stage (P < .001), tumor diameter (P = .040), and TPI expression (P = .028) were significantly associated with poor prognosis (Table 3). Kaplan–Meier analysis and log-rank test showed that the OS was significantly worse in high TPI expression group compared to low expression group (45.03 vs 72.85 months, n = 92, P = .02, Fig. 2A). In order to further analyze the influence of TPI expression in different clinical progression of GC patients, we also used Kaplan–Meier analysis to compare OS according to TPI expression in different depth of invasion, lymph node metastasis status, TNM stages, and tumor diameter, respectively. The results of Kaplan–Meier analysis showed that TPI at higher expression was also a significant predictor of decreased OS in tumor diameter >5 cm and the patients with positive lymph node metastasis (35.31 vs 65.89 months, n = 44, P = .018, Fig. 2B; 36.83 vs 71.75 months, n = 65, P = .022, Fig. 2C). When tumor diameter was <5 cm, and lymph node metastasis, depth of invasion, and TNM stages were negative, no significant differences were found between TPI high expression group and TPI low expression group. Next, PH was evaluated with the above variables. PH assumption indicated that these variables had not interactive effects on time, and they could be added into multivariable Cox PH models. In the multivariate analysis, TPI expression was an independent prognostic factor for OS after adjusting for TNM stage and tumor diameter (hazards ratio 3.112, 95% confidence interval 1.209–8.077, P < .05, Table 4). Finally, 3 variables, such as TNM stage, TPI, and tumor diameter, were added in a multivariate risk model to calculate the PI score. The score was calculated by the following equation:
Table 3.
Univariate analysis of overall survival for 92 GC patients.
Figure 2.
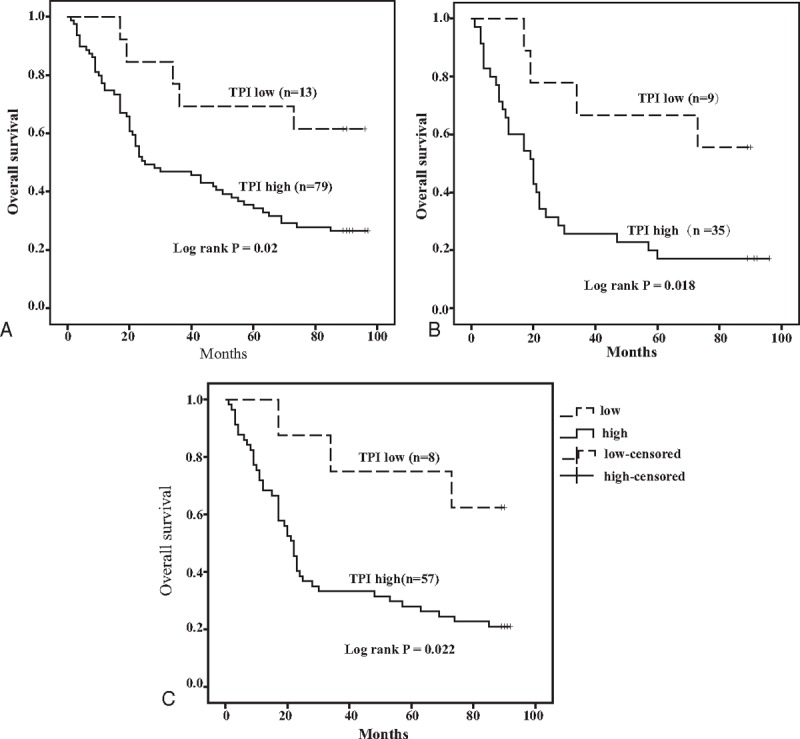
Kaplan–Meier analysis of GC patients’ OS curves. (A) OS curves of 92 GC patients with TPI low and high expression. Patients with TPI high expression had shorter OS than those with low (log-rank test, P = .020). (B) OS curves of 44 patients with tumor diameter >5 cm according to TPI levels (log-rank test, P = .018). (C) OS curves of 65 patients with positive lymph node metastasis according to TPI levels (log-rank test, P = .022). GC = gastric cancer, OS = odds ratio, TPI = triosephosphate isomerase.
Table 4.
Multivariate analysis of overall survival for 92 GC patients.
PIOS = 1.108 (0 or 1) + 0.698 (0 or 1) + 0.514 (0 or 1).
4. Discussion
Our present study was the first exploring TPI expression in GC tissue and the corresponding adjacent healthy tissues. We also analyzed the relationship between TPI expression and patients’ characteristics or clinicopathological features. In addition, we evaluated TPI role in GC patients’ prognosis.
TPI is highly expressed in various tumors and may play an important role in carcinogenesis progression, such as in esophageal cancer,[19] colorectal cancer,[20] pancreatic cancer,[21] and lung squamous cell carcinoma.[22] Similarly, in our study TPI was higher in GC tissues than in the corresponding adjacent healthy tissues. TPI may act as an oncogene, promoting proliferation, migration, and invasion of tumor cells, and it would become a cancer-related biomarker in certain tumors. Roth et al[23] established a unique cellular model of the adenoma-to-carcinoma sequence in colorectal cancer to study the progression of colorectal cancer from adenomas to carcinomas. They found that TPI was upregulated in carcinomas compared with adenomas, indicating that TPI was related with colorectal cancer development. TPI high expression in GC might have the same meaning. Gess et al[24] reported that TPI upregulation was due to HIF-1a in hypoxia environment either in vivo or in vitro. Under hypoxia, TPI mRNA amount was elevated in mouse hepatoma Hepa1 cells; however, it was changeless in Hepa1C4 since it could not form the active HIF-1a, suggesting that TPI upregulation may be triggered by an HIF-dependent pathway, with HIF-1a as the main trigger of TPI expression. According to Gess study, we speculated that high TPI expression in GC might be regulated by HIF-1a. Nevertheless, the correlation between high TPI expression in GC and HIF-1a needs to be verified.
TPI was found to be associated with gender. Indeed, TPI expression was higher in all female patients compared to male. To the best of our knowledge, no reports revealed the relationship between TPI expression and gender. We speculated that this result might be associated with the selection of the sample, such as different disease state and different age structure, leading to different results. However, no difference had been found between male and female in distribution of age and TNM stage when we made further analysis. The difference in TPI expression between the 2 genders might probably due to the limited number of cases. Further studies are needed to confirm that whether and why the expression of TPI is higher in female with GC than in male.
Our present study was the first revealing the relationship between TPI expression and GC patients prognosis. GC patients with higher TPI expression showed shorter OS than those with lower or negative expression. As those patients with the same clinicopathological characteristics could have divergent outcomes, evaluation of the effect of different TPI expression in different pathological progress of the disease might give a further risk stratification. When we stratified our patients by different lymph node metastasis status, those with high TPI expression had a shorter survival than those with low TPI expression in all positive lymph node metastatic patients. High TPI expression patients had a shorter survival than those with low TPI expression also in all negative lymph node metastatic patients, although no statistically significant difference was observed by log-rank text. Katayama et al[25] reported that TPI was significantly increased in SW620 cell line (colorectal cancer cell line) derived from a lymph node metastasis in comparison to the SW480 cell line derived from the primary lesion, suggesting that TPI may be associated with the metastatic process of these 2 cell lines to some extent. In this study, although no association between TPI expression and lymph node metastasis was observed, in the stratified analysis we found that high TPI expression in patients with positive lymph node metastasis corresponded with a worse prognosis, indicating that TPI role might be more significant in patients with positive lymph node metastasis. The multivariate analysis showed that high TPI expression was associated with poor prognosis. Thus, TPI might be a potential prognostic factor for GC survival. It is well known that p53 or HER-2 could be associated to chemotherapy efficacy and affect the OS in advanced GC patients.[14] The correlation between TPI, the newfound independent prognostic factor in GC, and P53 or HER-2, and the role of this correlation in the prognosis of GC mechanisms, needs more evidences in a larger sample size and more prospective study.
In addition, the multivariate analysis showed that TNM stage and tumor diameter were independent prognostic factors in GC patients. TNM stage is one of the acknowledged prognostic factors in GC patients. However, since tumor size plays an important role in prognostic factor in many malignancies, its prognostic value in GC has not been well defined.[26] Tumor size had been reported as an independent prognostic factor in diffuse-type advanced gastric cancer (AGC).[27] In our study, either univariate Cox regression analysis or multivariate analysis found that tumor size was a prognostic factor for OS. The stratified analyses showed that, in patients whose tumor size greater than 5 cm, those with high expression of TPI survived shorter than those with low (P < .05). However, no statistical difference was found between TPI high and low expression by log-rank test in all patients with tumor size less than 5 cm, suggesting that TPI prognostic significance is not clear in patients with tumor size less than 5 cm. TPI is a required enzyme in glycolysis. Tumor cells preferentially select aerobic glycolysis to gain energy, and the activity of glycolytic metabolism is beneficial to the survival and proliferation of malignancies.[28] A bigger tumor needs more nutrition to gain the required energy to perform a higher proliferation rate. This aspect could represent a possible explanation, to a certain extent, for the relationship between TPI expression and tumor size that we found.
5. Conclusion
Overall, our results revealed that TPI expression might be considered as a novel prognostic factor to evaluate GC patients’ survival.
Acknowledgments
The authors thank Scientific and Technological plans of Guangdong province, China (No: 2013B021800065), the Natural Science Fund Project of Guangdong province, China (No: 2016A030313683), and the Social Science and Technology Development Project of Dongguan, Guangdong province, China (No: 2016108101039) for the support.
Footnotes
Abbreviations: GC = gastric cancer, OS = overall survival, PH = proportional hazards, PI = prognostic index, TMA = tissue microarray, TNM = tumor node metastasis, TPI = triosephosphate isomerase.
TC and ZH contributed equally to this work.
Informed consent: Written approval consent was obtained from all patients included in this study.
Funding/support: This work was funded by the Scientific and Technological plans of Guangdong province, China (No: 2013B021800065), the Natural Science Fund Project of Guangdong province, China (No: 2016A030313683), and the Social Science and Technology Development Project of Dongguan, Guangdong province, China (No: 2016108101039).
The authors have no conflicts of interest to disclose.
References
- [1].Zhou C, Ji J, Cai Q, et al. MTA2 enhances colony formation and tumor growth of gastric cancer cells through IL-11. BMC Cancer 2015;15:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Meng X, Wang X, Ding C. The research progress of lymph node metastasis in gastric cancer. Chin Imaging J Integr Traditional Western Med 2015;13:90–2. [Google Scholar]
- [3].Liu X, Xu P, Qiu H, et al. Clinical utility of HER2 assessed by immunohistochemistry in patients undergoing curative resection for gastric cancer. Onco Targets Ther 2016;9:949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shi Y, Chen L, Wang Z, et al. Clinical and prognostic significances of VEGF, HIF-1α and TGF-β1 expressions for postoperative gastric cancer patients with stage I-III. Chin Clin Oncol 2013;4:310–6. [Google Scholar]
- [5].Ali AL, Donna MM, Jing L, et al. A gene-rich cluster between the CD4 and triosephosphate isomerase genes at human. Genome Res 1996;6:314–26. [DOI] [PubMed] [Google Scholar]
- [6].Judith RB, Ira D, James RK, et al. Characterization of the functional gene and several processed pseudogenes in the human triosephosphate isomerase gene family. Mol Cell Biol 1985;7:1694–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Maquat L, Chilcote R, Ryan P. Human triosephosphate isomerase cDNA and protein structure. Studies of triosephosphate isomerase deficiency in man. J Biol Chem 1985;260:37–48. [PubMed] [Google Scholar]
- [8].Chen GA, Gharib TG, Huang CC, et al. Proteomic analysis of lung adenocarcinoma: identification of a highly expressed set of proteins in tumors. Clin Cancer Res 2002;8:2298–305. [PubMed] [Google Scholar]
- [9].Montgaomerie JZ, Garcy RW, Holshuh HJ, et al. The 28K protein in urinary bladder, squamous metaplasia and urine is triosephosphate isomerase. Clin Biochem 1997;8:613–8. [DOI] [PubMed] [Google Scholar]
- [10].Tamesa MS, Kuramitsu Y, Fujimoto M, et al. Detection of autoantibodies against cyclophilin A and triosephosphate isomerase in sera from breast cancer patients by proteomic analysis. Electrophoresis 2009;30:2168–81. [DOI] [PubMed] [Google Scholar]
- [11].Wang JW, Peng SY, Li JT, et al. Identification of metastasis-associated proteins involved in gallbladder carcinoma metastasis by proteomic analysis and functional exploration of chloride intracellular channel 1. Cancer Lett 2009;281:71–81. [DOI] [PubMed] [Google Scholar]
- [12].Thongwatchara P, Promwikorn W, Srisomsap C, et al. Differential protein expression in primary breast cancer and matched axillary node metastasis. Oncol Rep 2011;26:185–91. [DOI] [PubMed] [Google Scholar]
- [13].Linge A, Susan K, Deirdre F, et al. Differential expression of fourteen proteins between uveal melanoma from patients who subsequently developed distant metastases versus those who did not. Biochem Mol Biol 2012;53:4634–42. [DOI] [PubMed] [Google Scholar]
- [14].Ren C, Wang W, Han C, et al. Expression and prognostic value of miR-92a in patients with gastric cancer. Tumor Biol 2016;37:9483–91. [DOI] [PubMed] [Google Scholar]
- [15].Giuseppe V, Daniele M, Simone A, et al. Short-term and long-term risk factors in gastric cancer. World J Gastroenterol 2015;21:6434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xu S, Huang J, Xu M, et al. ACK1 promotes gastric cancer epithelial-mesenchymal transition and metastasis through AKT-POU2F1-ECD signalling. J Pathol 2015;236:175–85. [DOI] [PubMed] [Google Scholar]
- [17].Yan-fang WU, Bo-yan Y, Ning LV, et al. Prognosis and clinicopathological characteristics of young patients with colorectal cancer: a retrospective analysis of 152 cases. Chin J Med 2015;7:36–9. [Google Scholar]
- [18].Chen F, Cao Y, Huang J, et al. A novel prognostic index for oral squamous cell carcinoma patients with surgically treated. Oncotarget 2017;doi:10.18632/oncotarget.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Qi YJ, He QY, Ma YF, et al. Proteomic identification of malignant transformation-related proteins in esophageal squamous cell carcinoma. J Cell Biochem 2008;104:1625–35. [DOI] [PubMed] [Google Scholar]
- [20].Roth U, Razawi H, Hommer J, et al. Differential expression proteomics of human colorectal cancer based on a syngeneic cellular model for the progression of adenoma to carcinoma. Proteomics 2010;10:194–202. [DOI] [PubMed] [Google Scholar]
- [21].Mikuriya K, Kuramitsu Y, Ryozawa S, et al. Expression of glycolytic enzymes is increased in pancreatic cancerous tissues as evidenced by proteomic profiling by two-dimensional electrophoresis and liquid chromatography-mass spectrometry/mass spectrometry. Int J Oncol 2007;30:849–55. [PubMed] [Google Scholar]
- [22].Kim JE, Koo KH, Kim YH, et al. Identification of potential lung cancer biomarkers using an in vitro carcinogenesis model. Exp Mol Med 2008;40:709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Roth U, Razawi H, Hommer J, et al. Differential expression proteomics of human colorectal cancer based on a syngeneic cellular model for the progression of adenoma to carcinoma. Proteomics 2010;10:194–202. [DOI] [PubMed] [Google Scholar]
- [24].Gess B, Hofbauer K, Deutzmann R, et al. Hypoxia up-regulates triosephosphate isomerase expression via a HIF-dependent pathway. Cell Mol Physiol 2004;448:175–80. [DOI] [PubMed] [Google Scholar]
- [25].Katayama M, Nakano H, Ishiuchi A, et al. Protein pattern difference in the colon cancer cell lines examined by two-dimensional differential in-gel electrophoresis and mass spectrometry. Surg Today 2006;36:1085–93. [DOI] [PubMed] [Google Scholar]
- [26].Liu H, Zhang H, Shen Z, et al. Increased expression of CSF-1 associates with poor prognosis of patients with gastric cancer undergoing gastrectomy. Medicine 2016;95:e2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yang J, Li L, Zhang G, et al. Clinical study on surgical method and prognosis in diffuse-type advanced gastric cancer. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2016;41:151–7. [DOI] [PubMed] [Google Scholar]
- [28].Kamarajugadda S, Stemboroski L, Cai Q, et al. Glucose oxidation modulates anoikis and tumor metastasis. Mol Cell Biol 2012;32:1893–907. [DOI] [PMC free article] [PubMed] [Google Scholar]


