Figure 2.
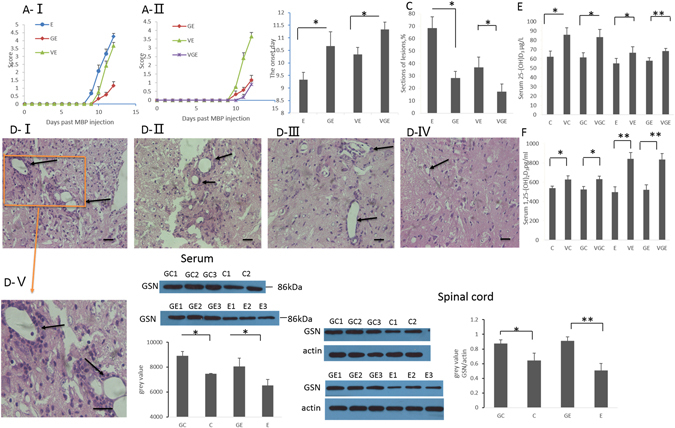
LV-GSN and vitamin D3 supplementation delays the onset of EAE and reduces inflammation. (A) Effects of LV-GSN, vitamin D3 and the combination of them on the course of EAE disease progression as evaluated by severity scoring. *p < 0.05. Shown is the composite mean from 17–18 mice/group evaluated in three separate experiments. (B) Average time of onset of EAE for rats fed with (VE) and without (E) vitamin D, injected with LV-GSN (GE), and injected with LV-GSN during vitamin D supplement (VGE) is shown. *p < 0.05. Shown is the composite mean from 17–18 mice/group evaluated in three separate experiments. (C) Statistical analysis of lesions in the spinal cord of E, GE, VE, and VGE rats. *p < 0.05. (D) Histopathological EAE disease in the spinal cord of E (D-I), VE (D-II), GE rats (D-III) and VGE rats (D-IV); D-Vis the higher magnification image of the marked part of D-I (representative images from 3 rats per group). The inflammatory lesions are indicated by arrows. (D-I, D-II, D-III, D-IV, ×200 magnification; D-V, ×400 magnification, scale bar 10 μm). (E) The concentration of 25-(OH) D3 in serum was measured by ELISA for rats fed without and with vitamin D supplementation in the control (C and VC), acute stage EAE (E and VE), and accompanied by LV-GSN (GE and VGE, GC, and VGC) groups. **p < 0.01, *p < 0.05. (F) ELISA was performed to measure the concentration of 1,25-(OH)2D3 in the serum of the same groups of rats as in panel E. **p < 0.01, *p < 0.05. (G) Western blot and real-time PCR was performed to detect the efficiency of LV-GSN in increasing the expression of GSN in serum. (H) The change of GSN level (protein and mRNA) in the spinal cord before (E) and after (GE) LV-GSN injection, *p < 0.05 Data are mean ± SD. **p < 0.01, *p < 0.05 (one-way ANOVA). The blots shown in Fig. 2G-I and 2H-I are cropped; the uncropped full-length gels are presented in the Supplementary Figure (Fig. S1C,D).
