Abstract
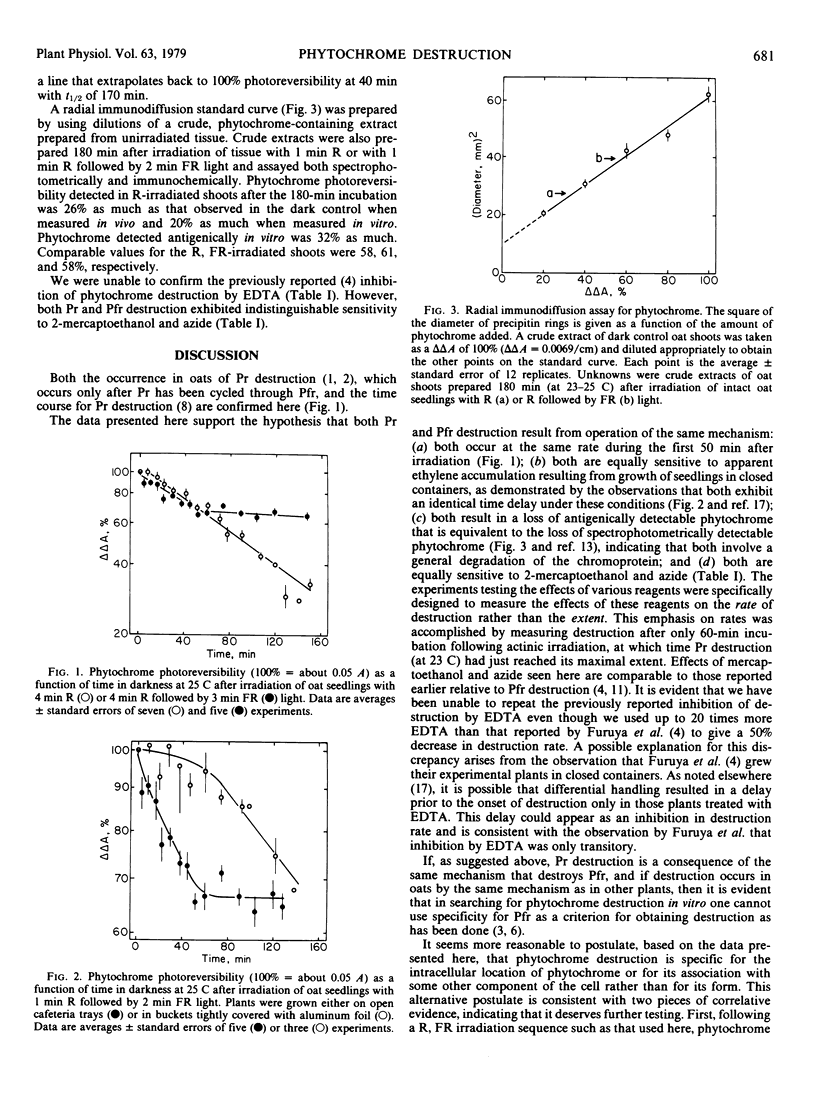
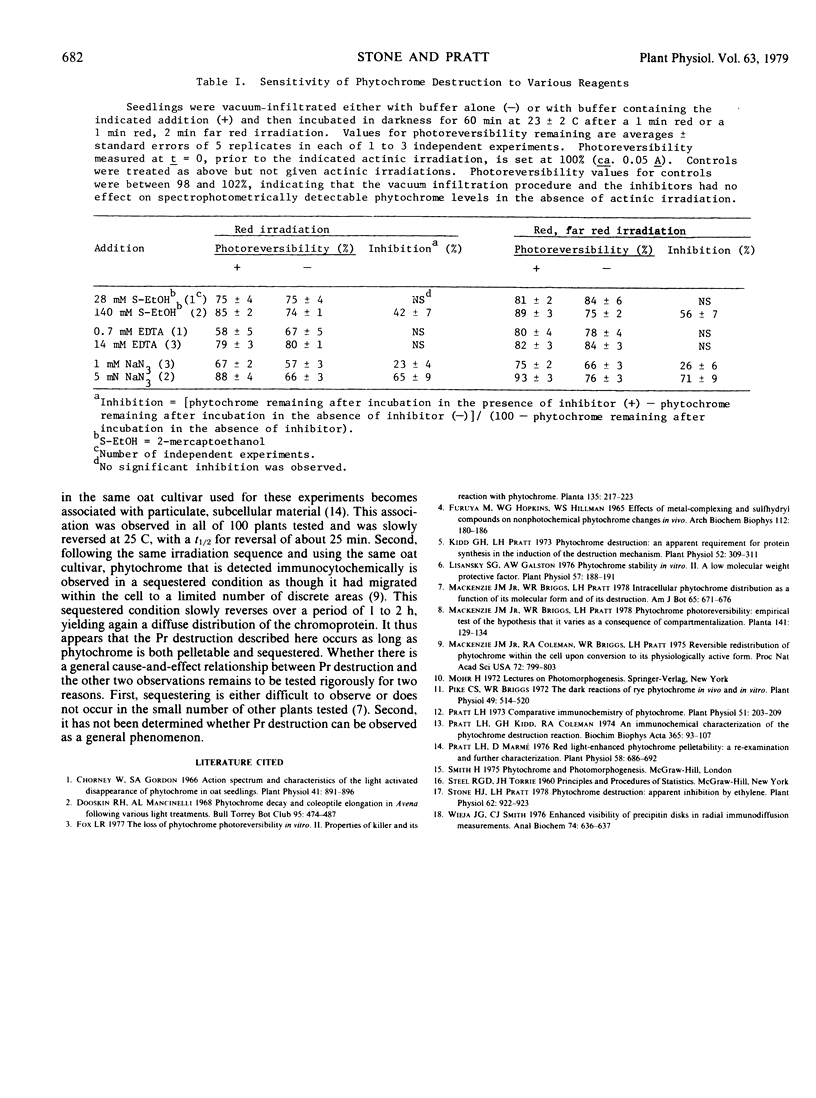
Both the red-absorbing (Pr) and far red-absorbing (Pfr) forms of phytochrome undergo destruction, defined as the loss of photoreversibly detectable chromoprotein following actinic irradiation of dark-grown tissue, in 4-day-old etiolated oat seedlings. Pr and Pfr destruction follow the same time course, exhibit the same time delay after actinic irradiation when the plants are grown in sealed containers, result in a loss of antigenically detectable phytochrome, as determined by radial immunodiffusion assay, equal to the loss of spectrophotometrically detectable phytochrome, and have the same sensitivity to 2-mercaptoethanol and azide. We suggest that Pr destruction is a consequence of the same mechanism that is responsible for Pfr destruction.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chorney W., Gordon S. A. Action spectrum and characteristics of the light activated disappearance of phytochrome in oat seedlings. Plant Physiol. 1966 May;41(5):891–896. doi: 10.1104/pp.41.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya M., Hopkins W. G., Hillman W. S. Effects of metal-complexing and sulfhydryl compounds on nonphotochemical phytochrome changes in vivo. Arch Biochem Biophys. 1965 Oct;112(1):180–186. doi: 10.1016/0003-9861(65)90026-3. [DOI] [PubMed] [Google Scholar]
- Kidd G. H., Pratt L. H. Phytochrome destruction: an apparent requirement for protein synthesis in the induction of the destruction mechanism. Plant Physiol. 1973 Oct;52(4):309–311. doi: 10.1104/pp.52.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisansky S. G., Galston A. W. Phytochrome Stability in Vitro: II. A Low Molecular Weight Protective Factor. Plant Physiol. 1976 Feb;57(2):188–191. doi: 10.1104/pp.57.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie J. M., Jr, Coleman R. A., Briggs W. R., Pratt L. H. Reversible redistribution of phytochrome within the cell upon conversion to its physiologically active form. Proc Natl Acad Sci U S A. 1975 Mar;72(3):799–803. doi: 10.1073/pnas.72.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike C. S., Briggs W. R. The dark reactions of rye phytochrome in vivo and in vitro. Plant Physiol. 1972 Apr;49(4):514–520. doi: 10.1104/pp.49.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. H. Comparative immunochemistry of phytochrome. Plant Physiol. 1973 Jan;51(1):203–209. doi: 10.1104/pp.51.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. H., Kidd G. H., Coleman R. A. An immunochemical characterization of the phytochrome destruction reaction. Biochim Biophys Acta. 1974 Sep 13;365(1):93–107. doi: 10.1016/0005-2795(74)90253-0. [DOI] [PubMed] [Google Scholar]
- Pratt L. H., Marmé D. Red Light-enhanced Phytochrome Pelletability: Re-examination and Further Characterization. Plant Physiol. 1976 Nov;58(5):686–692. doi: 10.1104/pp.58.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone H. J., Pratt L. H. Phytochrome destruction: apparent inhibition by ethylene. Plant Physiol. 1978 Dec;62(6):922–923. doi: 10.1104/pp.62.6.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieja J. G., Smith C. J. Enhanced visibility of precipitin disks in radial immunodiffusion measurements. Anal Biochem. 1976 Aug;74(2):636–637. doi: 10.1016/0003-2697(76)90252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


