Abstract
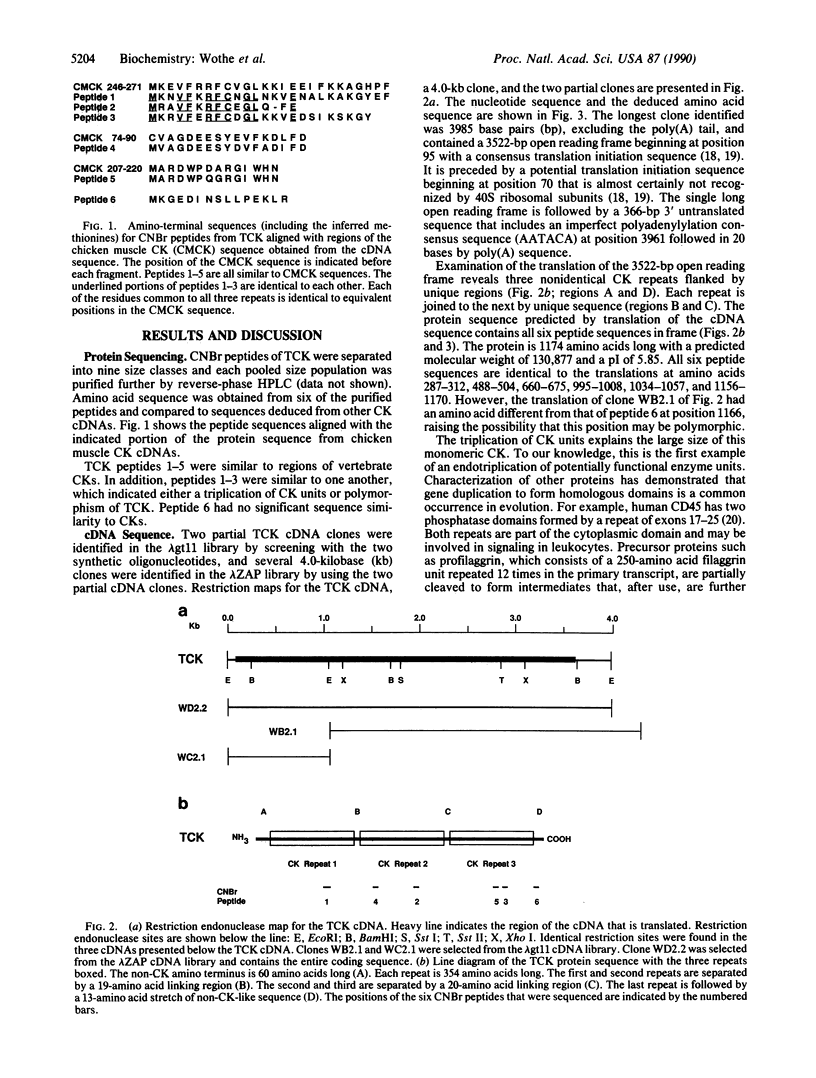
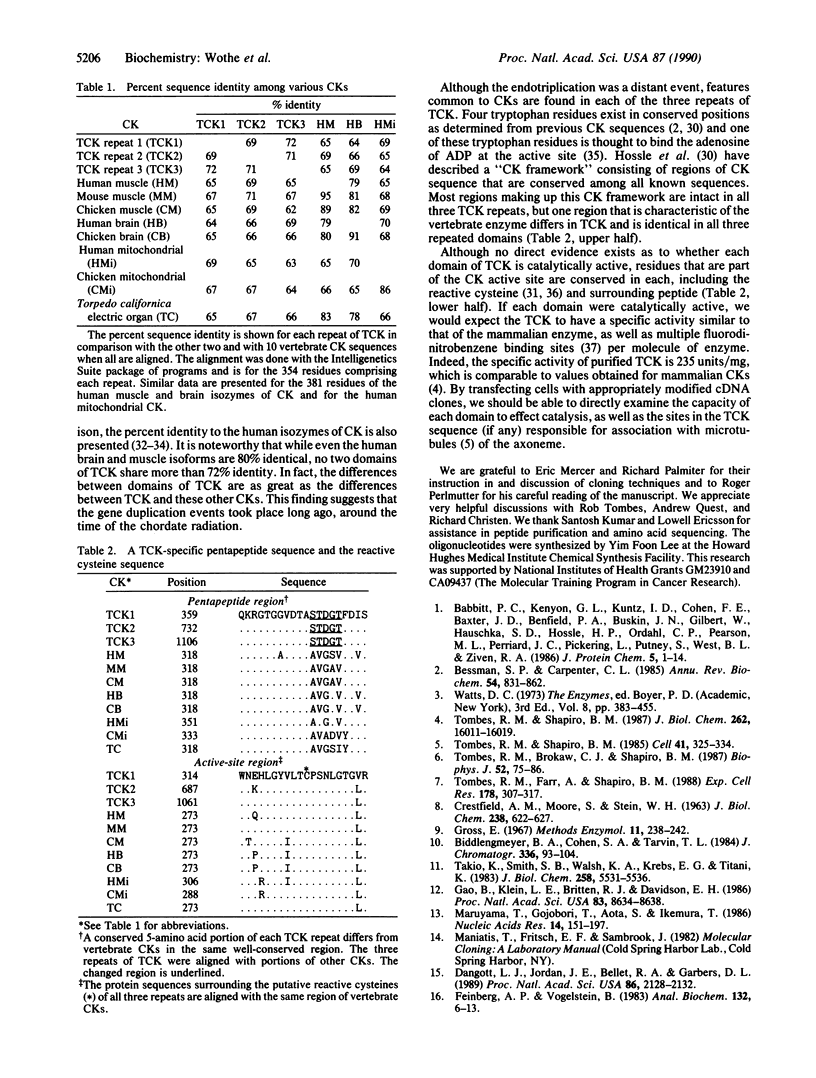
TCK, the creatine kinase (ATP:creatine N-phosphotransferase) from sperm flagella of the sea urchin Strongylocentrotus purpuratus, is a Mr 145,000 axonemal protein that is employed in energy transport. Its amino acid sequence was obtained by analysis of fragments from cyanogen bromide digestion and by sequencing cDNA clones from two sea urchin testis libraries. TCK contains three complete but nonidentical creatine kinase segments joined by regions of sequence that are not creatine kinase-like and flanked by unique amino and carboxyl termini. Each creatine kinase segment is homologous to vertebrate creatine kinases of both muscle and brain types, and all three repeats contain the essential active-site cysteine. The sequence differences among repeats suggest an ancient gene triplication, around the time of the chordate-echinoderm divergence. The echinoderm, with a unique creatine kinase in sperm, arginine kinase in eggs, and both phosphagen kinases in somatic cells, may represent a preserved branch point in evolution, and TCK may be a relic of this event.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessman S. P., Carpenter C. L. The creatine-creatine phosphate energy shuttle. Annu Rev Biochem. 1985;54:831–862. doi: 10.1146/annurev.bi.54.070185.004151. [DOI] [PubMed] [Google Scholar]
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Buskin J. N., Jaynes J. B., Chamberlain J. S., Hauschka S. D. The mouse muscle creatine kinase cDNA and deduced amino acid sequences: comparison to evolutionarily related enzymes. J Mol Evol. 1985;22(4):334–341. doi: 10.1007/BF02115689. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Dangott L. J., Jordan J. E., Bellet R. A., Garbers D. L. Cloning of the mRNA for the protein that crosslinks to the egg peptide speract. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2128–2132. doi: 10.1073/pnas.86.7.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gao B., Klein L. E., Britten R. J., Davidson E. H. Sequence of mRNA coding for bindin, a species-specific sea urchin sperm protein required for fertilization. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8634–8638. doi: 10.1073/pnas.83.22.8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas R. C., Korenfeld C., Zhang Z. F., Perryman B., Roman D., Strauss A. W. Isolation and characterization of the gene and cDNA encoding human mitochondrial creatine kinase. J Biol Chem. 1989 Feb 15;264(5):2890–2897. [PubMed] [Google Scholar]
- Hall L. R., Streuli M., Schlossman S. F., Saito H. Complete exon-intron organization of the human leukocyte common antigen (CD45) gene. J Immunol. 1988 Oct 15;141(8):2781–2787. [PubMed] [Google Scholar]
- Hossle J. P., Rosenberg U. B., Schäfer B., Eppenberger H. M., Perriard J. C. The primary structure of chicken B-creatine kinase and evidence for heterogeneity of its mRNA. Nucleic Acids Res. 1986 Feb 11;14(3):1449–1463. doi: 10.1093/nar/14.3.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossle J. P., Schlegel J., Wegmann G., Wyss M., Böhlen P., Eppenberger H. M., Wallimann T., Perriard J. C. Distinct tissue specific mitochondrial creatine kinases from chicken brain and striated muscle with a conserved CK framework. Biochem Biophys Res Commun. 1988 Feb 29;151(1):408–416. doi: 10.1016/0006-291x(88)90608-0. [DOI] [PubMed] [Google Scholar]
- Kaye F. J., McBride O. W., Battey J. F., Gazdar A. F., Sausville E. A. Human creatine kinase-B complementary DNA. Nucleotide sequence, gene expression in lung cancer, and chromosomal assignment to two distinct loci. J Clin Invest. 1987 May;79(5):1412–1420. doi: 10.1172/JCI112969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon G. L., Reed G. H. Creatine kinase: structure-activity relationships. Adv Enzymol Relat Areas Mol Biol. 1983;54:367–426. doi: 10.1002/9780470122990.ch6. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski R. W., Schweinfest C. W., Dottin R. P. Molecular cloning and the complete nucleotide sequence of the creatine kinase-M cDNA from chicken. Nucleic Acids Res. 1984 Sep 25;12(18):6925–6934. doi: 10.1093/nar/12.18.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAHOWALD T. A., NOLTMANN E. A., KUBY S. A. Studies on adenosine triphosphate transphosphorylases. III. Inhibition reactions. J Biol Chem. 1962 May;237:1535–1548. [PubMed] [Google Scholar]
- Nigro J. M., Schweinfest C. W., Rajkovic A., Pavlovic J., Jamal S., Dottin R. P., Hart J. T., Kamarck M. E., Rae P. M., Carty M. D. cDNA cloning and mapping of the human creatine kinase M gene to 19q13. Am J Hum Genet. 1987 Feb;40(2):115–125. [PMC free article] [PubMed] [Google Scholar]
- Oliviero S., DeMarchi M., Carbonara A. O., Bernini L. F., Bensi G., Raugei G. Molecular evidence of triplication in the haptoglobin Johnson variant gene. Hum Genet. 1985;71(1):49–52. doi: 10.1007/BF00295668. [DOI] [PubMed] [Google Scholar]
- Ordahl C. P., Evans G. L., Cooper T. A., Kunz G., Perriard J. C. Complete cDNA-derived amino acid sequence of chick muscle creatine kinase. J Biol Chem. 1984 Dec 25;259(24):15224–15227. [PubMed] [Google Scholar]
- Ratto A., Shapiro B. M., Christen R. Phosphagen kinase evolution. Expression in echinoderms. Eur J Biochem. 1989 Dec 8;186(1-2):195–203. doi: 10.1111/j.1432-1033.1989.tb15195.x. [DOI] [PubMed] [Google Scholar]
- Resing K. A., Walsh K. A., Haugen-Scofield J., Dale B. A. Identification of proteolytic cleavage sites in the conversion of profilaggrin to filaggrin in mammalian epidermis. J Biol Chem. 1989 Jan 25;264(3):1837–1845. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takio K., Smith S. B., Walsh K. A., Krebs E. G., Titani K. Amino acid sequence around a "hinge" region and its "autophosphorylation" site in bovine Lung cGMP-dependent protein kinase. J Biol Chem. 1983 May 10;258(9):5531–5536. [PubMed] [Google Scholar]
- Tombes R. M., Brokaw C. J., Shapiro B. M. Creatine kinase-dependent energy transport in sea urchin spermatozoa. Flagellar wave attenuation and theoretical analysis of high energy phosphate diffusion. Biophys J. 1987 Jul;52(1):75–86. doi: 10.1016/S0006-3495(87)83190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombes R. M., Farr A., Shapiro B. M. Sea urchin sperm creatine kinase: the flagellar isozyme is a microtubule-associated protein. Exp Cell Res. 1988 Oct;178(2):307–317. doi: 10.1016/0014-4827(88)90401-6. [DOI] [PubMed] [Google Scholar]
- Tombes R. M., Shapiro B. M. Energy transport and cell polarity: relationship of phosphagen kinase activity to sperm function. J Exp Zool. 1989 Jul;251(1):82–90. doi: 10.1002/jez.1402510110. [DOI] [PubMed] [Google Scholar]
- Tombes R. M., Shapiro B. M. Enzyme termini of a phosphocreatine shuttle. Purification and characterization of two creatine kinase isozymes from sea urchin sperm. J Biol Chem. 1987 Nov 25;262(33):16011–16019. [PubMed] [Google Scholar]
- Tombes R. M., Shapiro B. M. Metabolite channeling: a phosphorylcreatine shuttle to mediate high energy phosphate transport between sperm mitochondrion and tail. Cell. 1985 May;41(1):325–334. doi: 10.1016/0092-8674(85)90085-6. [DOI] [PubMed] [Google Scholar]
- Vasák M., Nagayama K., Wüthrich K., Mertens M. L., Kägi J. H. Creatine kinase. Nuclear magnetic resonance and fluorescence evidence for interaction of adenosine 5'-diphosphate with aromatic residue(s). Biochemistry. 1979 Nov 13;18(23):5050–5055. doi: 10.1021/bi00590a004. [DOI] [PubMed] [Google Scholar]
- West B. L., Babbitt P. C., Mendez B., Baxter J. D. Creatine kinase protein sequence encoded by a cDNA made from Torpedo californica electric organ mRNA. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7007–7011. doi: 10.1073/pnas.81.22.7007. [DOI] [PMC free article] [PubMed] [Google Scholar]



