Abstract
Background/Aim:
People suffering of diabetes increased significantly worldwide. Population, in Sub-Saharan Africa and mainly in Gabon, rely on medicinal plants to manage diabetes, as well in rural as in urban areas. This study aimed to survey a wide range of Gabonese plants for their antidiabetic activity.
Materials and Methods:
This study focused on the identification of medicinal plants used in the local treatment of diabetes mellitus. Ethnobotanical investigations were carried out in rural and urban areas of three provinces of Gabon using a semi-structured interview.
Results:
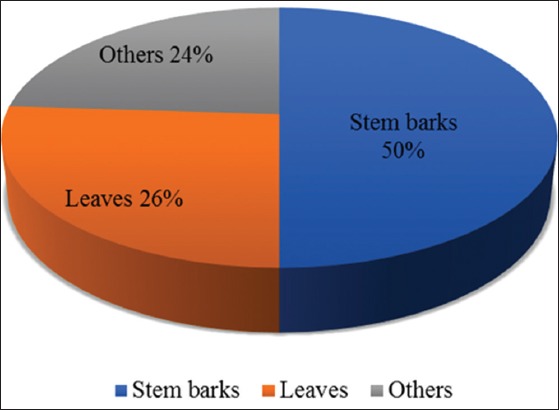
About 50 plant species belonging to 31 families and 50 genera were recorded, a majority of which have been documented previously to have medicinal properties. Most have documented antidiabetic properties with characterized therapeutic chemical compounds. Of the plant parts used for treatment, stem barks were employed most frequently (50%), followed by leaves (26%); the remaining 24% comprised roots, fibers, fruit, bulbs, flowers, rhizom, skin, and stem. Regarding the mode of preparation, decoction was the most widely used (58%), followed by maceration (18%) and infusion (14%). Almost all the plant products were administered orally (98%).
Conclusions:
Taken in concert, this study highlights the possibility of exploiting traditional knowledge of specific medicinal plants for the inexpensive treatment and management of diabetes.
KEY WORDS: Medical plants, Bio-efficacy, diabetes mellitus, ethnopharmacology, Gabon
INTRODUCTION
Diabetes mellitus is a metabolic disorder characterized by disruption of carbohydrate, fat, and protein metabolism. The disorder is associated with severe complications, including retinopathy, microangiopathy, and peripheral neuropathy [1]. Diabetes causes major economic losses worldwide and impedes country development [2,3].
The number of persons affected by diabetes is expected to reach 438.4 million worldwide in 2030 [4]. Only a fraction (49.3%) of the population in Africa has been tested for the disease [5] but, in sub-Saharan Africa alone, an estimated 10.4 million people lived with diabetes in 2007 [6]. In the central African country of Gabon, which has a population of ~1.7 million people [7], 10.71% of the population has been diagnosed with this disease [8]. Since, pharmaceutical products used for the management of diabetes are expensive for rural populations and may induce serious side effects [9], medicinal plants are used predominately to treat this disease. According to George et al. [10], medicinal plants contain biologically active compounds with diverse therapeutic applications. For example, saponins and alkaloids in Alstonia boonei De Wild. have a diuretic effect and are utilized in the treatment of urinary edema and hypertension [11]. The fungicidal action of saponins in (Piptadeniastrum africanum Hoof. f.) Brenan provides another example [12] used in traditional medicine. In Gabon, 78.2% of the species of plants in forests are used medicinally by pygmies [13], which exemplifies this country’s botanical medicinal heritage. It is important to improve understanding of plants used by local people in the treatment of diabetes in Gabon and which may have beneficial applications for the world at large. The aim of this study is to survey a wide range of Gabonese plants for their antidiabetic activity. Studies were performed in villages and towns across three provinces in Gabon that represent different types of rainforest.
MATERIALS AND METHODS
Study Area
Gabon is a small francophone country located in Central Africa bordering the Atlantic Ocean at the Equator between the Republic of the Congo and Equatorial Guinea. The climate is always hot and humid. Gabon houses some of Africa’s most biodiverse rainforests, which comprise approximately 80% of the country and stretch to the coast. Research in the Northwest and South Central/East of Gabon was done in the following three provinces: Estuaire (N.W. coastal region), Ogooué-Lolo (south-central forest region), and Haut-Ogooué (southeast mosaic of forest-savanna) [Figure 1]. The sampling was conducted in both rural areas and urban regions, including is even towns and six departments of the three provinces [Table 1].
Figure 1.
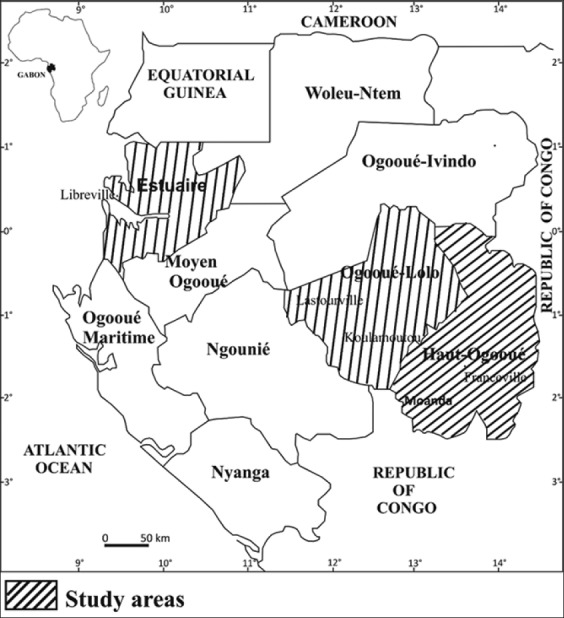
Map of study areas
Table 1.
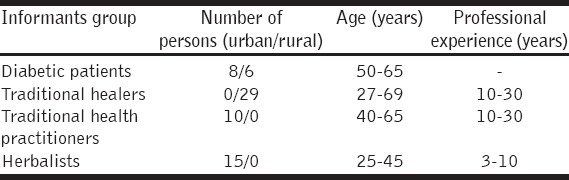
Demographic data of key informants
Investigation Method
The ethnobotanical survey was conducted between October 2014 and March 2015, which spans periods of sparse but heavy rainfall (October-November), a short dry season (December-January), and part of the long wet season with heavy rainfall (February-April). The investigation was carried out using a semi-structured questionnaire in French or in the native language of the informant. Interviewees included diabetic patients, traditional healers, traditional health practitioners, herbalists, and other knowledgeable people. The recorded parameters were locality, sociodemographic data (age and gender), vernacular or local plant names, plant parts used, method of preparation, method of administration, quantity consumed, and type of material, samples collected for botanical identification were dried, preserved and identified by an expert botanist, ISSEMBE Yves, at National Herbarium of Libreville, Gabon. The Latino names of some plant species have been updated using the plant list database [14].
Data Analysis
The frequency of citation (FC) of a plant species was evaluated using the following formula: FC = (Number of times a particular species was mentioned/Total number of times that all species were mentioned) × 100 [15,16].
RESULTS
Demographic Characteristics
A total of 80 people were investigated, of which 68 informants had a rich knowledge of herbal medicine [Table 2]. The balance did not report knowledge of medicinal plants and was excluded from further study. Of those that reported informations; 14 were patients with physician-diagnosed a diabetes mellitus or people were relatives of people suffering from diabetes, 29 were traditional healers, 10 were traditional health practitioners, and 15 were herbalists. More than half (65%) of the interviewees were male, and the average age of both sexes was approximatively 53 years with informants ranging in age until 70 years. More than half of all respondents (51.5%) were from rural areas, traditional healers who were the most numerous informants were mainly represented areas rural while herbalists and traditional health practitioners were only recorded that in urban areas.
Table 2.
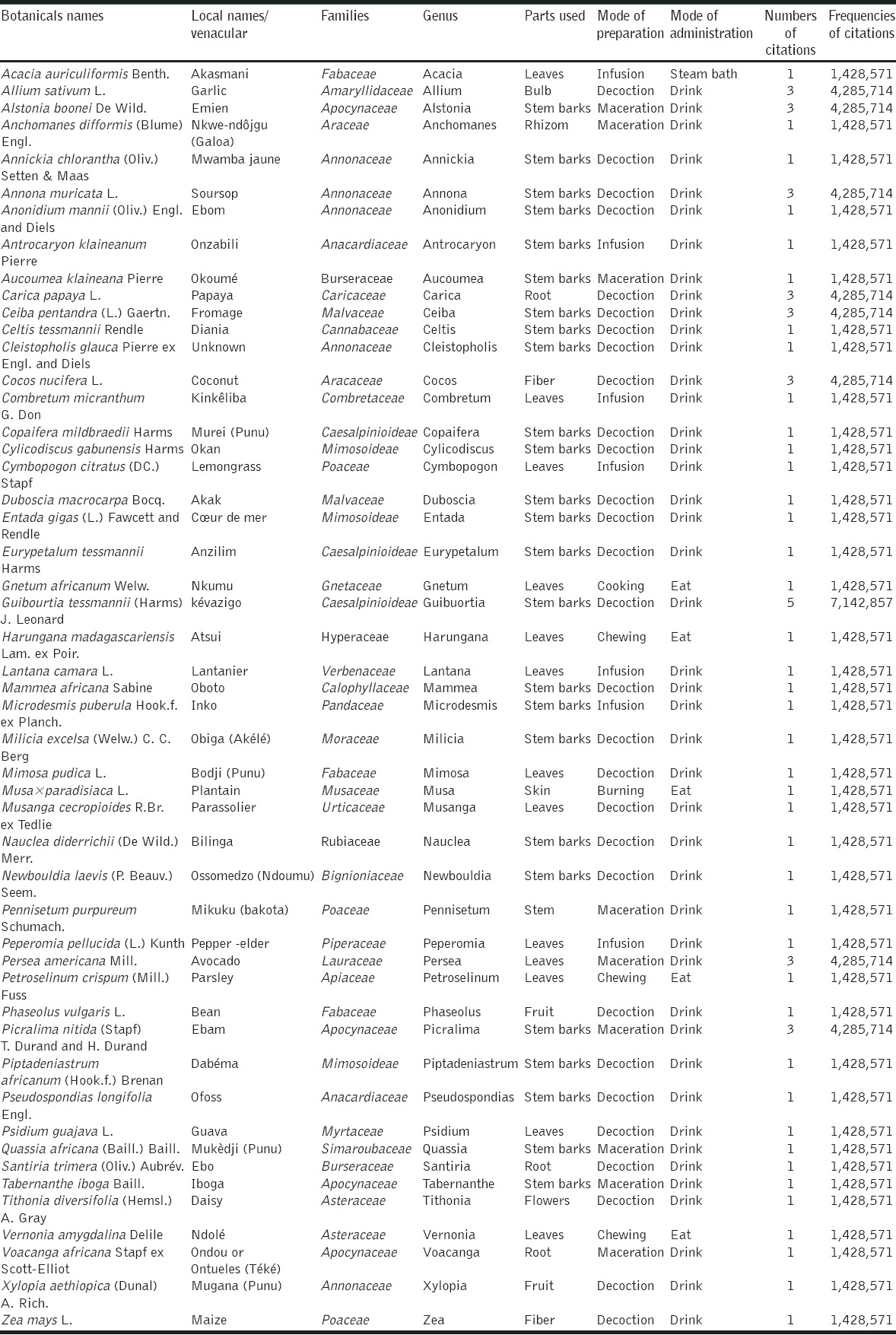
Data of medicinal plants traditionally used for the management diabetes mellitus
Ethnobotanical Characteristics and Associated Knowledge
The species cited by respondents in this study were listed in alphabetical order by scientific name, local or vernacular name, family, genus, plants parts used, mode of preparation, mode of administration, and FC [Table 2]. 50 species belonging to 31 families and 50 genus were used for the treatment of diabetes. The Annonaceae was the most commonly represented of all families [Figure 2], with particular use of soursop Annona muricata L. Nine plant species were most cited by interviewers as a remedy for diabetes, of which Guibourtia tessmannii (Harms) J. Leonard (Caesalpinioideae) was the most frequent (7.14%) followed by A. boonei (Apocyanceae), Carica papaya L. (Caricaceae), Persea americana Mill. (Lauraceae), Allium sativum L. (Amaryllidaceae), A. muricata (Annonaceae), Ceiba pentandra (L.) Gaertn. (Malvaceae), Cocos nucifera L. (Arecaceae), Picralima nitida (Stapf) T. Durand and H. Durand (Apocynaceae) (4.29%). The others species were least cited, it is the case of Annickia chlorantha (Oliv.) Setten and Maas (Annonaceae), Cymbopogon citratus (DC.) Stapf (Poaceae), Eurypetalum tessmannii Harms (Caesalpinioideae), Lantana camara L. (Verbenaceae), Musa × paradisiaca L. (Musaceae), Psidium guajava L. (Myrtaceae), Vernonia amygdalina Delile (Asteraceae), Xylopia aethiopica (Dunal) A. Rich. (Annonaceae), and the gymnosperm Gnetum africanum Welw. (Gnetaceae) [Table 2]. Bibliographic research showed that about 94% of plants were well-documented in literature [Table 3]. All 50 plants are used to prepare medicinal drugs individually or in various combinations.
Figure 2.
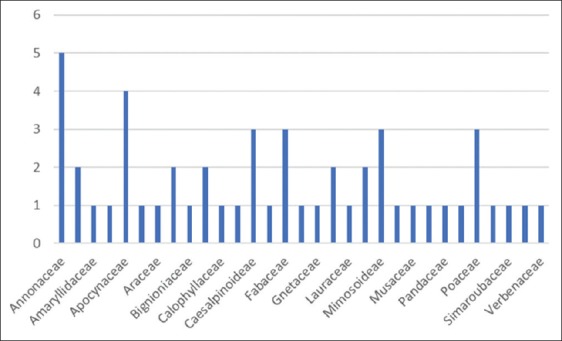
Repartition of plants families
Table 3.
Phytochemical and pharmacological properties of plants
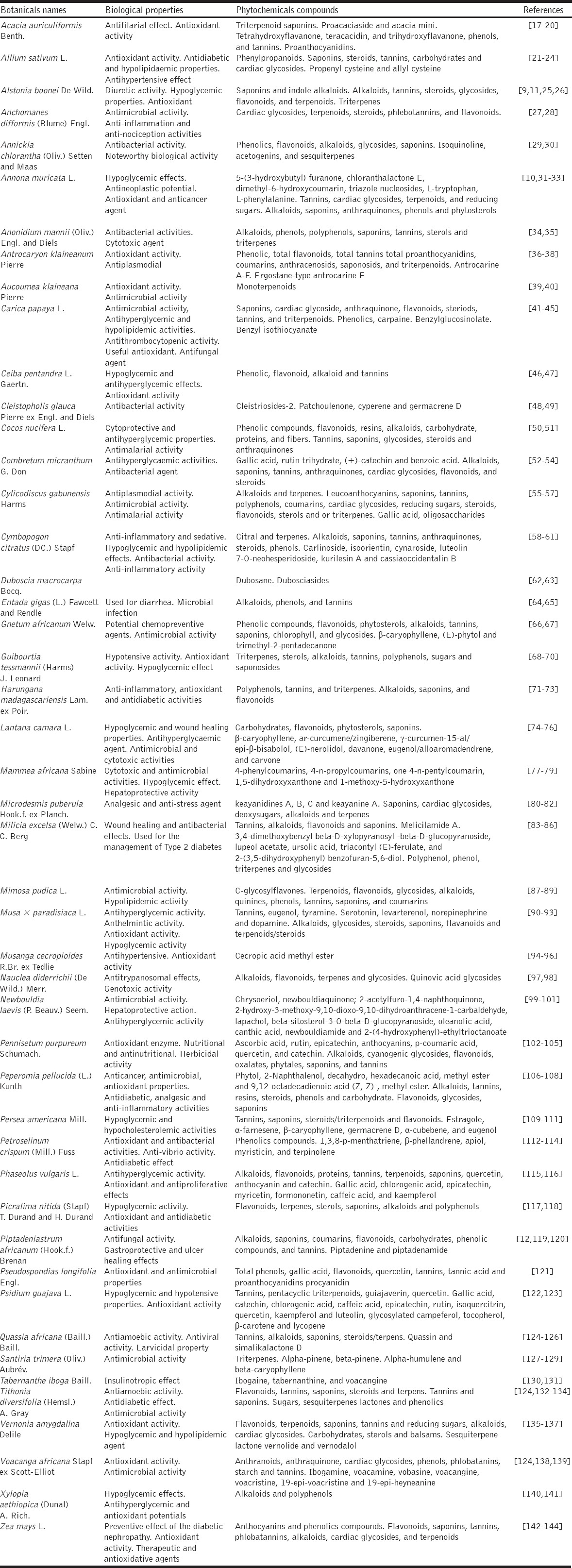
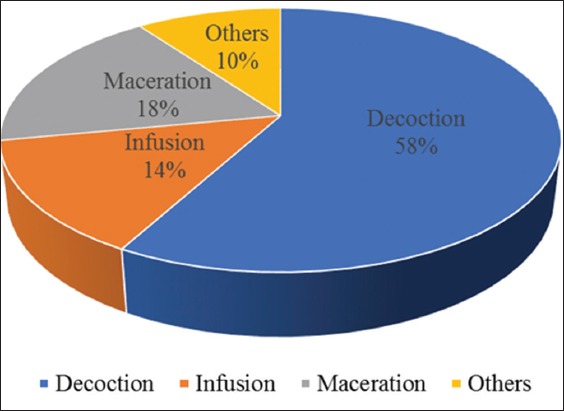
The result shows that the most frequently used plant parts were stem barks (50%) followed by leaves (26%) and other plant parts (24%), including roots (6%), fibers (4%), bulbs, fruit, flower, rhizom, skin, and stem (2% each) [Figure 3]. Most components were prepared by decoction (58%). Maceration (18%) and infusion (14%) were other modes of preparation and use, as was chewing (4%), burning and cooking (2%) [Figure 4]. Three modes of administration were used. Herbal products were primarily administered orally (98% of cases), mostly in liquid form (88%). Administration by mastication was also recorded (10% of cases) as was treatment by vapor bath (2% of cases) [Figure 5].
Figure 3.
Plant parts cited for treating diabetes in the same areas of Gabon
Figure 4.
Pharmaceutical forms used to treat diabetes in some Gabonese regions
Figure 5.
Mode of administration of recipes in the treatment of diabetes in some Gabonese regions
DISCUSSION
The results of demographic data showed that most knowledgeable interviewees were male (65%) of average age >50 years. A previous study found that women (69%) frequently used more medicinal plants than men (31%) [145]. Uniyal et al. [146] also found that men knew comparatively more about plant-based medicines than females because women were occupied by household working pressure. In Gabon, women tender house gardens and are more ready than men to bring out the first health care.
Respondents were dominated by aged people (>50 years). This experience is consistent with the study of Etuk et al. [147], in which showed the estimated age range of respondents was 40-70 years. Others have documented a profound and growing knowledge gap regarding medicinal plants between old and young people [148]. According to Uniyal et al. [146], the younger generations are ignorant of the vast medicinal resources available in their surroundings and are occupied in the search for money through market resources. Transmission of traditional medicinal knowledge from one generation to the next is thereby under threat [13,16].
It was also found that plant-based medicinal knowledge was more prevalent among people living in rural rather than urban area as described earlier by Vashistha [149]. Indeed, in a rural area, endogenous knowledges being more preserved [150], people resort, culturally, to the use of traditional medicine and herbal drugs are socioeconomically acceptable [151,152].
50 medicinal plants were exploited by both rural and urban people for the treatment of diabetes. Annonaceae was the most represented family. Members of the Annonaceae contain natural products with varied therapeutic properties, such as the anti flavonol taxifolin [153], which is known to possess antidiabetic, antitumor, and anti-inflammatory properties [154]. In addition, Annonaceae acetogenins are potent mitochondrial toxins with anticancer and anti-HIV activities [154]. However, excessive use of A. muricata has been associated with atypical parkinsonism on the island of Guadeloupe [155].
Among plant components used for medicinal purposes, stem barks were most often used followed by leaves in accord with the findings of other investigators [13,16,147]. Bark is easily collected and contains concentrated bioactive [58,60]. However, leaves which also accumulate pharmacologically active principles reportedly are often used to manage diabetes [15,156]. Whereas the collection of leaves does not induce plant damage, collection of bark, roots or the whole plant is destructive and may lead to species depletion [157]. Some respondents recognized and addressed this problem with a traditional ritual in which a coin was placed at the base of the tree and while the injured part was wiped with dead leaves. This practice reportedly was undertaken to facilitate a rapid regeneration of the excised part of the plant.
Herbal drugs were most commonly used as oral decoctions. This observed was in accordance with the work of Madingou et al., [68] who observed that healing plants are generally boiled in medicinal recipes and then taken orally by many healers in Gabon and also many other reports worldwide [158-160].
Evaluating the bio-efficacy of the medicinal plants recorded, it was observed that each plant was mentioned at least twice by people from different regions for the management of diabetes. The literature also reports the use of some of these plants for diabetes treatment in others countries such as A. boonei has been studied in Nigeria [9]; P. americana, studied in Nigeria and Brazil [109,110]; P. nitida in Nigeria and Cameroon [117,118].
Moreover, the literature reports antidiabetic properties of many of these plants. 15 of them would have hypoglycemic, hypolipemia the case of P. americana, P. guajava, C. citratus, C. pentandra, C. papaya, L. camara, A. muricata, and A. sativum [22,109,110]. C. pentandra would have both antihyperglycemic and hypoglycemic effects [46]. Guibourtia would have antioxidant and hypoglycemic [69,70]. Since, the frequency of plant use citations by both traditional healers and literature is an indication of the pharmacological relevance of the plant and thus, of curative properties [156], one may argue the therapeutic properties of some of the investigated medicinal plants which were evidenced by their studied pharmacological properties.
CONCLUSION
The study highlights the drug discovery great potential of the Congo Basin Forest. Nowadays, the management of diabetes is not the only fact of modern medicine, many medicinal based plants recipes are proposed by healers worldwide and deserve to be valued and rationalize.
ACKNOWLEDGMENTS
The authors wish to thank all local population for their collaboration during the field investigations, who shared their knowledge on the use of medicinal plants with us. Without their contribution, this study would have been impossible. Authors also thank Mr. Oscar Metandou and Rolland Mitola who accompanied them in the field surveys. Special thanks to the botanists of the National Herbarium of Gabon (NHG), Mr. Thomas Nzabi, Mr. Yves Issembe, Mr. Raoul Niangadouma and Mr. Davy Ikabanga for their expertise in botanical identification of plants. We are grateful to Dr. Stephan Padzys, Jake Lowenstein, Professor Nicola Mary Anthony (University of New Orleans) and Professor and Senior Scientist Peter Spencer (Oregon Health and Science University) for their kind help and suggestions regarding the English translation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Akhtar MS, Ali MR. Study of anti diabetic effect of a compound medicinal plant prescription in normal and diabetic rabbits. J Pak Med Assoc. 1984;34:239–44. [PubMed] [Google Scholar]
- 2.Chauhan A, Sharma PK, Srivastava P, Kumar N, Dudhe R. Plants having potential antidiabetic activity: A review. Pharm Lett. 2010;2:369–87. [Google Scholar]
- 3.Patel DK, Kumar R, Laloo D, Hemalatha S. Natural medicines from plant source used for therapy of diabetes mellitus: An overview of its pharmacological aspects. Asian Pac J Trop Dis. 2012;2:239–50. doi: 10.1016/S2221-1691(12)60067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mbanya JC, Motala AA, Sobngwi E, Assah FK, Enoru ST. Diabetes in sub-Saharan Africa. Lancet. 2010;375:2254–66. doi: 10.1016/S0140-6736(10)60550-8. [DOI] [PubMed] [Google Scholar]
- 5.Peer N, Kengne AP, Motala AA, Mbanya JC. Diabetes in the Africa Region: An update. Diabetes Res Clin Pract. 2014;103:197–205. doi: 10.1016/j.diabres.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Diabetes Atlas. IDF. 13rd ed. [[Last accessed on 2016 Jun 25]]. Available from: https://www.idf.org/sites/default/files/attachments/article_495_en.pdf .
- 7.Countrymeters Gabon Population. 2017. [[Last accessed on 2017 Mar 02]]. Available from: http://www.countrymeters.info/fr/Gabon .
- 8.Atlas du Diabète de la FID. 6e édition. 2014. [[Last accessed on 2015 Feb 12]]. Available from: http://www.idf.org/sites/default/files/Atlas-poster-2014_FR.pdf .
- 9.Akinloye OA, Oshilaja RT, Okelanfa OA, Akinloye DI, Idowu OM. Hypoglyceamic activity of Alstonia boonei stem bark extract in mice. Agric Biol J N Am. 2013;4:1–5. [Google Scholar]
- 10.George VC, Kumar DR, Rajkumar V, Suresh PK, Kumar RA. Quantitative assessment of the relative antineoplastic potential of the n-butanolic leaf extract of Annona muricata Linn in normal and immortalized human cell lines. Asian Pac J Cancer Prev. 2012;13:699–704. doi: 10.7314/apjcp.2012.13.2.699. [DOI] [PubMed] [Google Scholar]
- 11.Adebayo MA, Adeboye JO, Ajaiyeoba EO. Preliminary phytochemical investigation and diuretic studies of Alstonia boonei stem bark in male wistar rats. J Nat Remedies. 2004;4:179–82. [Google Scholar]
- 12.Brusotti G, Tosi S, Tava A, Picco AM, Grisoli P, Cesari I, et al. Antimicrobial and phytochemical properties of stem bark extracts from Piptadeniastrum africanum (Hook.f.) brenan. Ind Crops Prod. 2013;43:612–6. [Google Scholar]
- 13.Betti JL, Yongo OD, Mbomio DO, Iponga DM, Ngoye A. An ethnobotanical and floristical study of medicinal plants among the Baka Pygmies in the periphery of the Ipassa- biosphere reserve, Gabon. Eur J Med Plants. 2013;3:174–205. [Google Scholar]
- 14.The Plant List. Version 1. 2013. [[Last accessed on 2017 Feb 11]]. Available from: http://www.theplantlist.org .
- 15.Ocvirk S, Kistler M, Khan S, Talukder SH, Hauner H. Traditional medicinal plants used for the treatment of diabetes in rural and urban areas of Dhaka, Bangladesh - An ethnobotanical survey. J Ethnobiol Ethnomed. 2013;9:43. doi: 10.1186/1746-4269-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dey AK, Rashid MM, Millat MS, Rashid MM. Ethnobotanicals survey of medicinal plants used traditional health practitioners and indigenous people in different districts of Chittagongs division, Bangladesh. Int J Pharm Sci Invent. 2014;3:1–7. [Google Scholar]
- 17.Ghosh M, Babu SP, Sukul NC, Mahato SB. Antifilarial effect of two triterpenoid saponins isolated from Acacia auriculiformis. Indian J Exp Biol. 1993;31:604–6. [PubMed] [Google Scholar]
- 18.Garai S, Mahato SB. Isolation and structure elucidation of three triterpenoid saponins from Acacia auriculiformis. Phytochemistry. 1997;44:137–40. doi: 10.1016/s0031-9422(96)00399-8. [DOI] [PubMed] [Google Scholar]
- 19.Barry KM, Mihara R, Davies NW, Mitsunaga T, Mohammed CL. Polyphenols in Acacia mangium and Acacia auriculiformis heartwood with reference to heart rot susceptibility. J Wood Sci. 2005;51:615–21. [Google Scholar]
- 20.Sathya A, Siddhuraju P. Role of phenolics as antioxidants, biomolecule protectors and as anti-diabetic factors –Evaluation on bark and empty pods of Acacia auriculiformis. Asian Pac J Trop Med. 2012;5:757–65. doi: 10.1016/S1995-7645(12)60139-4. [DOI] [PubMed] [Google Scholar]
- 21.Ichikawa M, Ryu K, Yoshida J, Ide N, Kodera Y, Sasaoka T, et al. Identification of six phenylpropanoids from garlic skin as major antioxidants. J Agric Food Chem. 2003;51:7313–7. doi: 10.1021/jf034791a. [DOI] [PubMed] [Google Scholar]
- 22.Thomson M, Al-Amin ZM, Al-Qattan KK, Shaban LH, Ali M. Anti-diabetic and hypolipidaemic properties of garlic (Allium sativum) in streptozotocin-induced diabetic rats. Int J Diabetes Metab. 2007;15:108–15. [Google Scholar]
- 23.Mikail HG. Phytochemical screening, elemental analysis and acute toxicity of aqueous extract of Allium sativum L. bulbs in experimental rabbit. J Med Plants Res. 2010;4:322–6. [Google Scholar]
- 24.Matsutomo T, Ushijima M, Kodera Y, Nakamoto M, Takashima M, Morihara N, et al. Metabolomic study on the antihypertensive effect of S-1-propenylcysteine in spontaneously hypertensive rats using liquid chromatography coupled with quadrupole-Orbitrap mass spectrometry. J Chromatogr B. 2017;1046:147–55. doi: 10.1016/j.jchromb.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 25.Chime SA, Ugwuoke EC, Onyishi IV, Brown SA, Onunkwo GC. Formulation and evaluation of Alstonia boonei stem bark powder tablets. Indian J Pharm Sci. 2013;75:226–30. [PMC free article] [PubMed] [Google Scholar]
- 26.Obiagwu MO, Ihekwereme CP, Ajaghaku DL, Okoye FB. The useful medicinal properties of the root-bark extract of Alstonia boonei (Apocynaceae) may be connected to antioxidant activity. ISRN Pharmacol. 2014;2014:741478. doi: 10.1155/2014/741478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eneojo AS, Egwari LO, Mosaku TO. In vitro antimicrobial screening on Anchomanes difformis (Blume) engl leaves and rhizomes against selected pathogens of public health importance. Adv Biol Res. 2011;5:221–5. [Google Scholar]
- 28.Adebayo AH, John-Africa LB, Agbafor AG, Omotosho OE, Mosaku TO. Anti-nociceptive and anti-inflammatory activities of extract of Anchomanes difformis in rats. Pak J Pharm Sci. 2014;27:265–70. [PubMed] [Google Scholar]
- 29.Adesokan AA, Akanji MA, Yakubu MT. Antibacterial potentials of aqueous extract of Enantia chlorantha stem bark. Afr J Biotechnol. 2007;6:2502–5. [Google Scholar]
- 30.Talontsi FM, Lamshöft M, Douanla-Meli C, Kouam SF, Spiteller M. Antiplasmodial and cytotoxic dibenzofurans from Preussia sp. harboured in Enantia chlorantha Oliv. Fitoterapia. 2014;93:233–8. doi: 10.1016/j.fitote.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Adewole SO, Caxton-Martins EA. Morphological changes and hypoglycemic effects of Annona muricata Linn. (Annonaceae) leaf aqueous extract on pancreatic β-cells of streptozotocin-treated diabetic rats. Afr J Biomed Res. 2006;9:173–87. [Google Scholar]
- 32.Ge H, Dai J. Chemical constituents of an endophytic fungus from Annona muricata. Zhongguo Zhong Yao Za Zhi. 2010;35:3151–5. [PubMed] [Google Scholar]
- 33.Gavamukulya Y, Abou-Elella F, Wamunyokoli F, AEl-Shemy H. Phytochemical screening, anti-oxidant activity and in vitro anticancer potential of ethanolic and water leaves extracts of Annona muricata (Graviola) Asian Pac J Trop Med. 2014;7S1:S355–63. doi: 10.1016/S1995-7645(14)60258-3. [DOI] [PubMed] [Google Scholar]
- 34.Djeussi DE, Noumedem JA, Seukep JA, Fankam AG, Voukeng IK, Tankeo SB, et al. Antibacterial activities of selected edible plants extracts against multidrug-resistant Gram-negative bacteria. BMC Complement Altern Med. 2013;13:164. doi: 10.1186/1472-6882-13-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuete V, Fankam AG, Wiench B, Efferth T. Cytotoxicity and modes of action of the methanol extracts of six Cameroonian medicinal plants against multidrug-resistant tumor cells. Evid Based Complement Alternat Med. 2013;2013:285903. doi: 10.1155/2013/285903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sima OC, Obame EL, Ondo JP, Zong C, Nsi EE, Traore A. Ethnotherapy study, phytochemical screening and antioxidant activity of Antrocaryon klaineanum Pierre and Anthocleista nobilis G. Don. medicinal plants from Gabon. Inter J Advan Res. 2015;3:812–9. [Google Scholar]
- 37.Douanla PD, Tabopda TK, Tchinda AT, Cieckiewicz E, Frédérich M, Boyom FF, et al. Antrocarines A-F, antiplasmodial ergostane steroids from the stem bark of Antrocaryon klaineanum. Phytochem. 2015;117:521–6. doi: 10.1016/j.phytochem.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Fouokeng Y, Akak CM, Tala MF, Azebaze AG, Dittrich B, Vardamides JC, et al. The structure of antrocarine E, an ergostane isolated from Antrocaryon klaineanum Pierre (Anacardiaceae) Fitoterapia. 2017;117:61–4. doi: 10.1016/j.fitote.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Koudou J, Obame LC, Kumulungui BS, Edou P, Figueredo G, Chalchat JC, et al. Volatile constituents and antioxidant activity of Aucoumea klaineana Pierre essential oil. Afr J Pharm Pharmacol. 2009;3:323–6. [Google Scholar]
- 40.Obame LC, Bongui JB, Andzi BT, Ondo JP, Edou EP, Koudou J. Antifungal and antibacterial activities of Aucoumea klaineana Pierre essential oil from Gabon. VRI Phytomed. 2014;2:17–21. [Google Scholar]
- 41.Suresh K, Deepa P, Harisaranraj R, Vaira AV. Antimicrobial and phytochemical investigation of the leaves of Carica papaya L. Cynodon dactylon (L.) Pers., Euphorbia hirta L., Melia azedarach L. and Psidium guajava L. Ethnobot Lealf. 2008;12:1184–91. [Google Scholar]
- 42.Maniyar Y, Bhixavatimath P. Antihyperglycemic and hypolipidemic activities of aqueous extract of Carica papaya Linn. leaves in alloxan-induced diabetic rats. J Ayurveda Integr Med. 2012;3:70–4. doi: 10.4103/0975-9476.96519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zunjar V, Dash RP, Jivrajani M, Trivedi B, Nivsarkar M. Antithrombocytopenic activity of carpaine and alkaloidal extract of Carica papaya Linn. leaves in busulfan induced thrombocytopenic wistar rats. J Ethnopharmacol. 2016;18:20–5. doi: 10.1016/j.jep.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 44.Castro-Vargas HI, Baumann W, Parada-Alfonso F. Valorization of agroindustrial wastes: Identification by LC-MS and NMR of benzylglucosinolate from papaya (Carica papaya L.) seeds, a protective agent against lipid oxidation in edible oils. Electrophoresis. 2016;37:1930–7. doi: 10.1002/elps.201500499. [DOI] [PubMed] [Google Scholar]
- 45.He X, Ma Y, Yi G, Wu J, Zhou L, Guo H, et al. Chemical composition and antifungal activity of Carica papaya Linn. seed essential oil against Candida spp. Lett Appl Microbiol. 2017 doi: 10.1111/lam.12711. [DOI] [PubMed] [Google Scholar]
- 46.Satyaprakash RJ, Rajesh MS, Bhanumathy M, Harish MS, Shivananda TN, Shivaprasad HN, et al. Hypoglycemic and antihyperglycemic effect of Ceiba pentandra L. gaertn in normal and streptozotocin-induced diabetic rats. Ghana Med J. 2013;47:121–7. [PMC free article] [PubMed] [Google Scholar]
- 47.Ravi Kiran C, Rao DB, Sirisha N, Rao TR. Assessment of phytochemicals and antioxidant activities of raw and germinating Ceiba pentandra (Kapok) seeds. J Biomed Res. 2015;29:414–9. doi: 10.7555/JBR.27.20120145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z, Wang P, Ding N, Song G, Li Y. Total synthesis of cleistetroside-2, partially acetylated dodecanyl tetrarhamnoside derivative isolated from Cleistopholis patens and Cleistopholis glauca. Carbohydr Res. 2007;342:1159–68. doi: 10.1016/j.carres.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 49.Ouattar ZA, Boti JB, Ahibo CA, Tomi F, Casanova J, Bighelli A. Combined analysis of the root bark oil of Cleistopholis glauca by chromatographic and spectroscopic techniques. Nat Prod Commun. 2013;8:1773–6. [PubMed] [Google Scholar]
- 50.Renjith RS, Chikku AM, Rajamohan T. Cytoprotective, antihyperglycemic and phytochemical properties of Cocos nucifera (L.) inflorescence. Asian Pac J Trop Med. 2013;6:804–10. doi: 10.1016/S1995-7645(13)60142-X. [DOI] [PubMed] [Google Scholar]
- 51.Balogun EA, Malomo SO, Adebayo JO, Ishola AA, Soladoye AO, Olatunji LA, et al. In vivo antimalarial activity and toxicological effects of methanolic extract of Cocos nucifera (Dwarf red variety) husk fibre. J Integr Med. 2014;12:504–11. doi: 10.1016/S2095-4964(14)60054-6. [DOI] [PubMed] [Google Scholar]
- 52.Chika A, Bello SO. Antihyperglycaemic activity of aqueous leaf extract of Combretum micranthum (Combretaceae) in normal and alloxan-induced diabetic rats. J Ethnopharmacol. 2010;129:34–7. doi: 10.1016/j.jep.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 53.TouréA Xu X, Michel T, Bangoura M. In vitro antioxidant and radical scavenging of Guinean kinkeliba leaf (Combretum micranthum G. Don) extracts. Nat Prod Res. 2011;25:1025–36. doi: 10.1080/14786419.2010.482048. [DOI] [PubMed] [Google Scholar]
- 54.Osonwa UE, Umeyor CE, Okon UV, Uronnachi EM, Nwakile CD. Stability studies on the aqueous extract of the fresh leaves of Combretum micranthum G. don used as antibacterial agent. J Chem Chem Eng. 2012;6:417–24. [Google Scholar]
- 55.Okokon JE, Ita BN, Udokpoh AE. Antiplasmodial activity of Cylicodiscus gabunensis. J Ethnopharmacol. 2006;107:175–8. doi: 10.1016/j.jep.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Kouitcheu ML, Kouam J, Penlap BV, Ngadjui BT, Fomum ZT, Etoa FX. Evaluation of antimicrobial activity of the stem bark of Cylicodiscus gabunensis (Mimosaceae) Afr J Tradit Complement Altern Med. 2007;4:87–93. doi: 10.4314/ajtcam.v4i1.31197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aldulaimi O, Uche FI, Hameed H, Mbye H, Ullah I, Drijfhout F, et al. A characterization of the antimalarial activity of the bark of Cylicodiscus gabunensis Harms. J Ethnopharmacol. 2017;198:221–5. doi: 10.1016/j.jep.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 58.Negrelle RR, Gomes EC. Cymbopogon citratus (DC.) Stapf: Chemical composition and biological activities. Rev Bras Planta Med. 2007;9:80–92. [Google Scholar]
- 59.Adeneye AA, Agbaje EO. Hypoglycemic and hypolipidemic effects of fresh leaf aqueous extract of Cymbopogon citratus Stapf. in rats. J Ethnopharmacol. 2007;112:440–4. doi: 10.1016/j.jep.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 60.Asaolu MF, Oyeyemi OA, Olanlokun JO. Chemical compositions, phytochemical constituents and in vitro biological activity of various extracts of Cymbopogon citratus. Pak J Nutr. 2009;8:1920–2. [Google Scholar]
- 61.Costa G, Ferreira JP, Vitorino C, Pina ME, Sousa JJ, Figueiredo IV, et al. Polyphenols from Cymbopogon citratus leaves as topical anti-inflammatory agents. J Ethnopharmacol. 2016;178:222–8. doi: 10.1016/j.jep.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 62.Wafo P, Kamdem RS, Ali Z, Anjum S, Khan SN, Begum A, et al. Duboscic acid: A potent a-glucosidase inhibitor with an unprecedented triterpenoidal carbon skeleton from Duboscia macrocarpa. Org Lett. 2010;12:5760–3. doi: 10.1021/ol1026552. [DOI] [PubMed] [Google Scholar]
- 63.Tchuendem MH, Douanla PD, Tabopda TK, Tchinda AT, Tamze V, Nkengfack AE, et al. Two new glycosides from Duboscia macrocarpa Bocq. Phytochem Lett. 2014;10:1–4. [Google Scholar]
- 64.Ariwaodo JO, Adeniji KA, Onadeji OM, Shasanya OS. Survey of wild plant seeds and their value in traditional herbal medicine in Osun State, Nigeria. J Res Forest Wildl Environ. 2013;4:38–51. [Google Scholar]
- 65.Fankam AG, Kuiate JR, Kuete V. Antibacterial activities of Beilschmiedia obscura and six other Cameroonian medicinal plants against multi-drug resistant Gram-negative phenotypes. BMC Complement Altern Med. 2014;14:241. doi: 10.1186/1472-6882-14-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iweala EE. Preliminary qualitative screening for cancer chemopreventive agents in Telfairia occidentalis Hook.f Gnetum africanum Welw., Gongronema latifolium Benth. and Ocimum gratissimum L. from Nigeria. J Med Food. 2009;1:58–63. [Google Scholar]
- 67.Edet UU, Ehiabhi OS, Ogunwande IA, Ekundayo O. Analyses of the volatile constituents and antimicrobial activities of Gongronema latifolium (Benth.) and Gnetum africanum L. J Essent Oil Bear Plants. 2013;8:324–9. [Google Scholar]
- 68.Madingou NO, Souza A, Lamidi M, Mengome LE, Mba EM, Bayissi B, et al. Study of medidcinal plants used in the management of cardiovascular disease at Libreville (Gabon): An ethnopharmacological approach. Int J Pharm Sci Res. 2012;3:111–9. [Google Scholar]
- 69.Nyangono BC, Tsague M, Ngondi JL, Oben JE. In vitro antioxidant activity of Guibourtia tessmannii Harms, J. Leonard (Caesalpinoidae) J Med Plants Res. 2013;7:3081–8. [Google Scholar]
- 70.Madingou NO, Traore A, Souza A, Mounanga MM, Samseny RR, Ouedraogo S, et al. Preliminary studies of acute and sub-chronic toxicity of the aqueous extract of Guibourtia tessmannii (Harms) J Leonard stem barks (Caesalpiniaceae) in mice and rats. Asian Pac J Trop Biomed. 2016;6:506–10. [Google Scholar]
- 71.Iwalewa EO, Adewale IO, Taiwo BJ, Arogundade T, Osinowo A, Daniyan OM, et al. Effects of Harungana madagascariensis stem bark extract on the antioxidant markers in alloxan induced diabetic and carrageenan induced inflammatory disorders in rats. J Complement Integr Med. 2008 DOI:10.2202/1553-3840.1088. [Google Scholar]
- 72.Momo CE, Ngwa AF, Dongmo GI, Oben JE. Antioxidant properties and α - amylase inhibition of Terminalia superba, Albizia Sp., Cola nitida, Cola odorata and Harungana madagascarensis used in the management of diabetes in Cameroon. J Health Sci. 2009;55:732–8. [Google Scholar]
- 73.Antia BS, Ita BN, Udo UE. Nutrient composition and in vitro antioxidant Properties of Harungana madagascariensis stembark extracts. J Med Food. 2015;18:609–14. doi: 10.1089/jmf.2014.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dash GK, Suresh P, Ganapaty S. Studies on hypoglycaemic and wound healing activities of Lantana camara Linn. J Nat Remed. 2001;1:105–10. [Google Scholar]
- 75.Venkatachalam T, Kumar VK, Selvi PK, Maske AO, Anbarasan V, Kumar PS. Antidiabetic activity of Lantana camara Linn fruits in normal and streptozotocin-induced diabetic rats. J Pharm Res. 2011;4:1550–2. [Google Scholar]
- 76.Satyal P, Crouch RA, Monzote L, Cos P, Awadh Ali NA, et al. The chemical diversity of Lantana camara: Analyses of essential oil samples from Cuba, Nepal, and Yemen. Chem Biodivers. 2016;13:336–42. doi: 10.1002/cbdv.201500271. [DOI] [PubMed] [Google Scholar]
- 77.Ouahouo BM, Azebaze AG, Meyer M, Bodo B, Fomum ZT, Nkengfack AE. Cytotoxic and antimicrobial coumarins from Mammea africana. Ann Trop Med Parasitol. 2004;98:733–9. doi: 10.1179/000349804X3126. [DOI] [PubMed] [Google Scholar]
- 78.Tchamadeu MC, Dzeufiet PD, Nouga CC, Azebaze AG, Allard J, Girolami JP, et al. Hypoglycaemic effects of Mammea africana (Guttiferae) in diabetic rats. J Ethnopharmacol. 2010;127:368–72. doi: 10.1016/j.jep.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 79.Okokon JE, Bawo MB, Mbagwu HO. Hepatoprotective activity of Mammea africana ethanol stem bark extract. Avicenna J Phytomed. 2016;6:248–59. [PMC free article] [PubMed] [Google Scholar]
- 80.Roumy V, Hennebelle T, Zamblé A, ZambléYao J, Sahpaz S, Bailleul F. Letter: Characterisation and identification of spermine and spermidine derivatives in Microdesmis keayana and Microdesmis puberula roots by electrospray ionisation tandem mass spectrometry and high-performance liquid chromatography/electrospray ionisation tandem mass spectrometry. Eur J Mass Spectrom (Chichester) 2008;14:111–5. doi: 10.1255/ejms.910. [DOI] [PubMed] [Google Scholar]
- 81.Okany CC, Ishola IO, Ashorobi RB. Evaluation of analgesic and antistress potential of methanolic stem wood extract of Microdesmis puberula Hook.f ex planch (Pandaceae) in mice. Int J Appl Res Nat Prod. 2012;5:30–6. [Google Scholar]
- 82.Okon AE, Otu IS, Adaeze OK, Godwin DK, Ndem JI, Fidelis UA. Phytochemical screening and effect of ethanol root extract of Microdesmis puberula on some haematological and biochemical parameters in normal male albino Wistar rats. J Med Plant Res. 2013;7:2338–42. [Google Scholar]
- 83.Udegbunam SO, Nnaji TO, Udegbunam RI, Okafor JC, Agbo I. Evaluation of herbal ointment formulation of Milicia excelsa (Welw) C.C berg for wound healing. Afr J Biotechnol. 2013;12:3351–9. [Google Scholar]
- 84.Hussain H, Nyongha AT, Dongo E, Ahmed I, Zhang W. Melicilamide A: A new ceramide from Milicia excelsa. Nat Prod Res. 2013;27:1246–9. doi: 10.1080/14786419.2012.724416. [DOI] [PubMed] [Google Scholar]
- 85.Ouete JL, Sandjo LP, Kapche DW, Yeboah SO, Mapitse R, Abegaz BM, et al. Excelsoside: A new benzylic diglycoside from the leaves of Milicia excelsa. Z Naturforsch C. 2014;69(7-8):271–5. doi: 10.5560/znc.2014-0087. [DOI] [PubMed] [Google Scholar]
- 86.Dzeufiet PD, Tchamadeu MC, Bilanda DC, Ngadena YS, Poumeni MK, Nana D, et al. Preventive effect of Milicia excelsa (Moraceae) aqueous extract on dexamethasone induced insulin resistance in rat. J Pharm Pharm Sci. 2014;5:1232–41. [Google Scholar]
- 87.Yuan K, Lü JL, Yin MW. Chemical constituents of C-glycosylflavones from Mimosa pudica. Yao Xue Xue Bao. 2006;41:435–8. [PubMed] [Google Scholar]
- 88.Gandhiraja N, Sriram S, Meenaa V, Srilakshmi JK, Sasikumar C, Rajeswari R. Phytochemical screening and antimicrobial activity of the plant extracts of Mimosa pudica L. against selected microbes. Ethnobot Lealf. 2009;13:618–24. [Google Scholar]
- 89.Rajendran R, Krishnakumar E. Hypolipidemic activity of chloroform extract of Mimosa pudica leaves. Avicenna J Med Biotechnol. 2010;2:215–21. [PMC free article] [PubMed] [Google Scholar]
- 90.Mallick C, Chatterjee K, Guhabiswas M, Ghosh D. Antihyperglycemic effects of separate and composite extract of root of Musa paradisiaca and leaf of Coccinia indica in streptozotocin-induced diabetic male albino rat. Afr J Tradit Complement Altern Med. 2007;4:362–71. doi: 10.4314/ajtcam.v4i3.31230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hussain A, Khan MN, Sajid MS, Iqbal Z, Khan MK, Abbas RZ, et al. In vitro screening of the leaves of Musa paradisiaca for anthelmintic activity. J Anim Plant Sci. 2010;20:5–8. [Google Scholar]
- 92.Mahmood A, Ngah N, Omar MN. Phytochemicals constituent and antioxidant activities in Musa x paradisiaca flower. Eur J Sci Res. 2011;66:311–8. [Google Scholar]
- 93.Sundaram SC, Subramanian S. Biochemical evaluation of hypoglycemic activity of Musa paradisiaca (Plantain) flowers in STZ induced experimental diabetes in rats. Asian J Res Chem. 2011;4:827–33. [Google Scholar]
- 94.Lontsi D, Sondengam BL, Ayafor JF, Tsoupras MG, Tabacchi R. Further triterpenoids of Musanga cecropioides: The structure of cecropic acid. Planta Med. 1990;56:287–9. doi: 10.1055/s-2006-960959. [DOI] [PubMed] [Google Scholar]
- 95.Adeneye AA, Ajagbonna OP, Adeleke TI, Bello SO. Preliminary toxicity and phytochemical studies of the stem bark aqueous extract of Musanga cecropioides in rats. J Ethnopharmacol. 2006;105:374–9. doi: 10.1016/j.jep.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 96.Tchouya GR, Nantia EA. Phytochemical analysis, antioxidant evaluation and total phenolic content of the leaves and stem bark of Musanga cecropioides R.Br. ex tedlie (Cecropiaceae), growing in Gabon. J Pharmacogn Phytochem. 2015;3:192–5. [Google Scholar]
- 97.Nwodo NJ, Agbo MO. Antitrypanosomal effects of methanolic extracts of Nuclea diderrichii (Merr.) and Spathodea campanulata stem bark. J Pharm Allied Sci. 2010 DOI:10.4314/JOPHAS.V7I5.63466. [Google Scholar]
- 98.Liu W, Di Giorgio C, Lamidi M, Elias R, Ollivier E, De Méo MP. Genotoxic and clastogenic activity of saponins extracted from Nauclea bark as assessed by the micronucleus and the comet assays in Chinese hamster ovary cells. J Ethnopharmacol. 2011;137:176–83. doi: 10.1016/j.jep.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 99.Kuete V, Eyong KO, Folefoc GN, Beng VP, Hussain H, Krohn K, et al. Antimicrobial activity of the methanolic extract and of the chemical constituents isolated from Newbouldia laevis. Pharmazie. 2007;62:552–6. [PubMed] [Google Scholar]
- 100.Hassan SW, Tillo MK, Lawal M, Umar RA, Ndakotsu MA, Farouk UZ, et al. Hepatoprotective action of stem extracts of Newbouldia laevis in rats treated with carbon tetrachloride (CCL4) J Global Biosci. 2015;4:1627–46. [Google Scholar]
- 101.Osigwe CC, Akah PA, Nworu CS, Okoye TC, Tchimene MK. Antihyperglycemic studies on the leaf extract and active fractions of Newbouldia laevis (Bignoniaceae) Pharmacol Pharm. 2015;6:518–32. [Google Scholar]
- 102.Yu HM, Wang BS, Chu HL, Chang LW, Yen WJ, Lin CJ, Duh PD, Napiergrass (Pennisetum purpureum S) protects oxidative damage of biomolecules and modulates antioxidant enzyme activity. Food Chem. 2007;105:1364–74. [Google Scholar]
- 103.Akaraonye CC, Ikewuchi JC. Nutritional and antinutritional components of (Pennisetum purpureum Schumach) Pak J Nutr. 2009;8:32–8. [Google Scholar]
- 104.Prinsen P, Gutierrez A, del Río JC. Lipophilic extractives from the cortex and pith of elephant grass (Pennisetum purpureum Schumach.) stems. J Agric Food Chem. 2012;60:6408–17. doi: 10.1021/jf301753w. [DOI] [PubMed] [Google Scholar]
- 105.Norhafizah MZ, Ismail BS, Chuah TS. Herbicidal activity of Pennisetum purpureum (Napier grass) Afr J Biotechnol. 2012;11:6269–73. [Google Scholar]
- 106.Wei LS, Wee W, Siong JY, Syamsumir DF. Characterization of anticancer, antimicrobial, antioxidant properties and chemical compositions of Peperomia pellucida leaf extract. Acta Med Iran. 2011;49:670–4. [PubMed] [Google Scholar]
- 107.Oloyede GK, Onocha PA, Olaniran BB. Phytochemical, toxicity, antimicrobial and antioxidant screening of leaf extracts of Peperomia pellucida from Nigeria. Adv Environ Biol. 2011;5:3700–9. [Google Scholar]
- 108.de Fátima Arrigoni-Blank M, Dmitrieva EG, Franzotti EM, Antoniolli AR, Andrade MR, Marchioro M. Anti-inflammatory and analgesic activity of Peperomia pellucida (L.) HBK (Piperaceae) J Ethnopharmacol. 2004;91:215–8. doi: 10.1016/j.jep.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 109.Brai BI, Odetola AA, Agomo PU. Hypoglycemic and hypocholesterolemic potential of Persea americana leaf extracts. J Med Food. 2007;10:356–60. doi: 10.1089/jmf.2006.291. [DOI] [PubMed] [Google Scholar]
- 110.Lima CR, Vasconcelos CF, Costa-Silva JH, Maranhão CA, Costa J, Batista TM, et al. Anti-diabetic activity of extract from Persea americana Mill. leaf via the activation of protein kinase B (PKB/Akt) in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2012;141:517–25. doi: 10.1016/j.jep.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 111.García-Rodríguez YM, Torres-Gurrola G, Meléndez-González C, Espinosa-García FJ. Phenotypic variations in the foliar chemical profile of Persea americana Mill cv. Hass. Chem Biodivers. 2016;13:1767–75. doi: 10.1002/cbdv.201600169. [DOI] [PubMed] [Google Scholar]
- 112.Wong PY, Kitts DD. Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chem. 2006;97:505–15. [Google Scholar]
- 113.Snoussi M, Dehmani A, Noumi E, Flamini G, Papetti A. Chemical composition and antibiofilm activity of Petroselinum crispum and Ocimum basilicum essential oils against Vibrio spp. strains. Microb Pathog. 2016;90:13–21. doi: 10.1016/j.micpath.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 114.Abou Khalil NS, Abou-Elhamd AS, Wasfy SI, El Mileegy IM, Hamed MY, Ageely HM. Antidiabetic and antioxidant impacts of desert date (Balanites aegyptiaca) and parsley (Petroselinum sativum) aqueous extracts: Lessons from experimental rats. J Diabetes Res. 2016;2016:8408326. doi: 10.1155/2016/8408326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Atchibri AL, Brou KD, Kouakou TH, Kouadio YJ, Gnakri D. Screening for antidiabetic activity and phytochemical constituents of common bean (Phaseolus vulgaris L.) seeds. J Med Plant Res. 2010;4:1757–61. [Google Scholar]
- 116.Ombra MN, d'Acierno A, Nazzaro F, Riccardi R, Spigno P, Zaccardelli M, et al. Phenolic composition and antioxidant and antiproliferative activities of the extracts of twelve common bean (Phaseolus vulgaris L.) endemic ecotypes of Southern Italy before and after cooking. Oxid Med Cell Longev. 2016;2016:1398298. doi: 10.1155/2016/1398298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nwakile CD, Okore VC. Picralima nitida seed oil I: Hypoglycemic activity. J Adv Pharm Educ Res. 2011;2:147–50. [Google Scholar]
- 118.Teugwa CM, Mejiato PC, Zofou D, Tchinda BT, Boyom FF. Antioxidant and antidiabetic profiles of two African medicinal plants: Picralima nitida (Apocynaceae) and Sonchus oleraceus (Asteraceae) BMC Complement Altern Med. 2013;13:175. doi: 10.1186/1472-6882-13-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ateufack G, Domgnim Mokam EC, Mbiantcha M, Dongmo Feudjio RB, David N, Kamanyi A, et al. Gastroprotective and ulcer healing effects of Piptadeniastrum Africanum on experimentally induced gastric ulcers in rats. BMC Complement Altern Med. 2015;15:214. doi: 10.1186/s12906-015-0713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dawé A, Mbiantcha M, Fongang Y, Nana WY, Yakai F, Ateufack G, et al. Piptadenin, a novel 3,4-secooleanane triterpene and piptadenamide, a New ceramide from the stem bark of Piptadeniastrum africanum (Hook.f.) brenan. Chem Biodivers. 2017:14. doi: 10.1002/cbdv.201600215. [DOI] [PubMed] [Google Scholar]
- 121.Obiang CS, Ondo JP, Atome GR, Engonga LC, Siawaya JF, Emvo EN. Phytochemical screening, antioxidant and antimicrobial potential of stem barks of Coula edulis Baill. Pseudospondias longifolia Engl. and Carapa klaineana Pierre. from Gabon. Asian Pac J Trop Med. 2016;6:557–63. [Google Scholar]
- 122.Ojewole JA. Hypoglycemic and hypotensive effects of Psidium guajava Linn. (Myrtaceae) leaf aqueous extract. Methods Find Exp Clin Pharmacol. 2005;27:689–95. doi: 10.1358/mf.2005.27.10.948917. [DOI] [PubMed] [Google Scholar]
- 123.Araújo HM, Rodrigues FF, Costa WD, Nonato Cde F, Rodrigues FF, Boligon AA, et al. Chemical profile and antioxidant capacity verification of Psidium guajava (Myrtaceae) fruits at different stages of maturation. EXCLI J. 2015;14:1020–30. doi: 10.17179/excli2015-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tona L, Kambu K, Ngimbi N, Cimanga K, Vlietinck AJ. Antiamoebic and phytochemical screening of some Congolese medicinal plants. J Ethnopharmacol. 1998;61:57–65. doi: 10.1016/s0378-8741(98)00015-4. [DOI] [PubMed] [Google Scholar]
- 125.Apers S, Cimanga K, Van den Berghe D, Van Meenen E, Longanga AO, Foriers A, et al. Antiviral activity of simalikalactone D, a quassinoid from Quassia africana. Planta Med. 2002;68:20–4. doi: 10.1055/s-2002-19870. [DOI] [PubMed] [Google Scholar]
- 126.Sama W, Ajaiyeoba EO, Choudhary MI. Larvicidal properties of simalikalactone D from Quassia africana (Simaroubaceae) baill and baill, on the malaria vector Anopheles gambiae. Afr J Tradit Complement Altern Med. 2014;11:84–8. [PMC free article] [PubMed] [Google Scholar]
- 127.Da Silva MF, Francisco RH, Gray AI, Lechat JR, Waterman PG. Lanost-7-en triterpenes from stem bark of Santiria trimera. Phytochem. 1990;29:1629–32. [Google Scholar]
- 128.Martins AP, Salgueiro LR, Gonçalves MJ, Proença da Cunha A, Vila R, Cañigueral S. Essential oil composition and antimicrobial activity of Santiria trimera bark. Planta Med. 2003;69:77–9. doi: 10.1055/s-2003-37025. [DOI] [PubMed] [Google Scholar]
- 129.Bikanga R, Makani T, Agnaniet H, Obame LC, Abdoul-Latif FM, Lebibi J, et al. Chemical composition and biological activities of Santiria trimera (Burseraceae) essential oils from Gabon. Nat Prod Commun. 2010;5:961–4. [PubMed] [Google Scholar]
- 130.Akendengue B, Lemamy GJ, Bourobou HB, Laurens A. Bioactive natural compounds from medico-magic plants of Bantu area. Stud Nat Prod Chem. 2005;32:803–20. [Google Scholar]
- 131.Souza A, Mbatchi B, Herchuelz A. Induction of insulin secretion by an aqueous extract of Tabernanhte iboga Baill. (Apocynaceae) in rat pancreatic islets of Langerhans. J Ethnopharmacol. 2011;133:1015–20. doi: 10.1016/j.jep.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 132.Miura T, Nosaka K, Ishii H, Ishida T. Antidiabetic effect of Nitobegiku, the herb Tithonia diversifolia, in KK-Ay diabetic mice. Biol Pharm Bull. 2005;28:2152–4. doi: 10.1248/bpb.28.2152. [DOI] [PubMed] [Google Scholar]
- 133.Ogundare AO. Antimicrobial effect of Tithonia diversifolia and Jatropha gossypifolia leaf extracts. Trends Appl Sci Res. 2007;2:145–50. [Google Scholar]
- 134.Sampaio BL, Edrada-Ebel RA, Da Costa FB. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: A model for environmental metabolomics of plants. Sci Rep. 2016 doi: 10.1038/srep29265. DOI:10.1038/srep29265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ayoola GA, Coker HA, Adesegun SA, Adepoju-Bello AA, Obaweya K, Ezennia EC, et al. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in southwestern Nigeria. Trop J Pharm Res. 2008;7:1019–24. [Google Scholar]
- 136.Akah PA, Alemji JA, Salawu OA, Okoye TC, Offiah NV. Effects of Vernonia amygdalina on biochemical and hematological parameters in diabetic rats. Asian J Med Sci. 2009;1:108–13. [Google Scholar]
- 137.Abay SM, Lucantoni L, Dahiya N, Dori G, Dembo EG, Esposito F, et al. Plasmodium transmission blocking activities of Vernonia amygdalina extracts and isolated compounds. Malar J. 2015;14:288. doi: 10.1186/s12936-015-0812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Duru CM, Onyedineke NE. In vitro antimicrobial assay and phytochemical analysis of ethanolic extracts of Voacanga africana seeds. J A Sci. 2010;6:119–22. [Google Scholar]
- 139.Chen HM, Yang YT, Li HX, Cao ZX, Dan XM, Mei L. Cytotoxic monoterpenoid indole alkaloids isolated from the barks of Voacanga africana Staph. Nat Prod Res. 2016;30:1144–9. doi: 10.1080/14786419.2015.1046132. [DOI] [PubMed] [Google Scholar]
- 140.Ogbonnia S, Adekunle AA, Bosa MK, Enwuru VN. Evaluation of acute and subacute toxicity of Alstonia congensis Engler (Apocynaceae) bark and Xylopia aethiopica (Dunal) A. Rich (Annonaceae) fruits mixtures used in the treatment of diabetes. Afr J Biotechnol. 2008;7:701–5. [Google Scholar]
- 141.Ezeja MI, Nwaehujor CO, Anaga AO. Antihyperglycaemic and in vitro antioxidant activities of Xylopia aethiopica fruit methanol extract. J Ethnopharmacol. 2016;35:1070–7. [Google Scholar]
- 142.Suzuki R, Okada Y, Okuyama T. The favorable effect of style of Zea mays L. on streptozotocin induced diabetic nephropathy. Biol Pharm Bull. 2005;28:919–20. doi: 10.1248/bpb.28.919. [DOI] [PubMed] [Google Scholar]
- 143.Lopez-Martinez LX, Oliart-Ros RM, Valerio-Alfaro G, Lee CH, Parkin KL, Garcia HS. Antioxidant activity, phenolic compounds and anthocyanins content of eighteen strains of Mexican maize. Food Sci Technol. 2009;42:1187–92. [Google Scholar]
- 144.Solihah MA, Wan Rosli WI, Nurhanan AR. Phytochemicals screening and total phenolic content of Malaysian Zea mays hair extracts. Int Food Res J. 2012;19:1533–8. [Google Scholar]
- 145.Eddouks M, Maghrani M, Lemhadri A, Ouahidi M-L, Jouad H. Ethnopharmacological survey of medicinal plants used for the treatment of diabetes mellitus, hypertension and cardiac diseases in the South-East region of Morocco (Tafilalet) J Ethnopharmacol. 2002;82:97–103. doi: 10.1016/s0378-8741(02)00164-2. [DOI] [PubMed] [Google Scholar]
- 146.Uniyal SK, Singh KN, Jamwal P, Lal B. Traditional use of medicinal plants among the tribal communities of Chhota Bhangal, Western Himalaya. J Ethnobiol Ethnomed. 2006;2:14. doi: 10.1186/1746-4269-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Etuk EU, Bello SO, Isezuo SA, Mohammed BJ. Ethnobotanical survey of medicinal plants used for the treatment of diabetes mellitus in the North-Western region of Nigeria. Asian J Exp Biol Sci. 2010;1:55–9. [Google Scholar]
- 148.Caniago I, Siebert SF. Medicinal plant ecology, knowledge and conservation in Kalimantan, Indonesia. Econ Bot. 1998;52:229–50. [Google Scholar]
- 149.Vashistha PB. An ethnobotanical study of plains of Yamuna Nagar district, Haryana, India. Int J Innov Res Sci Eng Technol. 2015;4:18600–7. [Google Scholar]
- 150.Vihotogbe-Sossa CN, Akissoe NH, Anihouvi VB, Ahohuendo BC, Ahanchede A, Sanni A, et al. Endogenous knowledge of four leafy vegetables used by rural populations in Benin. Ecol Food Nutr. 2012;51:22–39. doi: 10.1080/03670244.2012.635570. [DOI] [PubMed] [Google Scholar]
- 151.Kolling M, Winkley K, von Deden M. “For someone who's rich, it's not a problem”. Insights from Tanzania on diabetes health-seeking and medical pluralism among Dar es Salaam's urban poor. Global Health. 2010;6:8. doi: 10.1186/1744-8603-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mangambu MJ, Mushagalusa KF, Kadima NJ. Contribution àl'étude phytochimique de quelques plantes médicinales antidiabétiques de la ville de Bukavu et ses environs (sud-Kivu, R.D. Congo) J Appl Biosci. 2014;75:6211–20. [Google Scholar]
- 153.Coria-Téllez AV, Montalvo-Gònzalez E, Yahia E M, Obledo-Vazquez EN. Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arab J Chem. 2016 DOI:10.1016/j.arabjc.2016.01.004. [Google Scholar]
- 154.Aminimoghadamfarouj N, Nematollahi A, Wiart C. Annonaceae: Bio-resource for tomorrow's drug discovery. J Asian Nat Prod Res. 2011;13:465–76. doi: 10.1080/10286020.2011.570265. [DOI] [PubMed] [Google Scholar]
- 155.Lannuzel A, Höglinger GU, Verhaeghe S, Gire L, Belson S, Escobar-Khondiker M, et al. Atypical parkinsonism in Guadeloupe: A common risk factor for two closely related phenotypes?Brain. 2007;130:816–27. doi: 10.1093/brain/awl347. [DOI] [PubMed] [Google Scholar]
- 156.Megersa M, Asfaw Z, Kelbessa E, Beyene A, Woldeab B. An ethnobotanical study of medicinal plants in Wayu Tuka district, East Welega zone of Oromia regional state, West Ethiopia. J Ethnobiol Ethnomed. 2013;9:68. doi: 10.1186/1746-4269-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schippmann U, Leaman JD, Cunningham AB. Impact of Cultivation and Gathering of Medicinal Plants on Biodiversity: Global Trends and Issues. Rome, Italy: FAO; 2002. pp. 1–21. [Google Scholar]
- 158.Erasto P, Adebola PO, Grierson DS, Afolayan AJ. An ethnobotanical study of plants used for the treatment of diabetes in the Eastern Cape province, South Africa. Afr J Biotechnol. 2005;4:1458–60. [Google Scholar]
- 159.Semenya S, Potgieter M, Erasmus L. Ethnobotanical survey of medicinal plants used by Bapedi healers to treat diabetes mellitus in the Limpopo province, South Africa. J Ethnopharmacol. 2012;141:440–5. doi: 10.1016/j.jep.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 160.Tsabang N, Fokou PV, Tchokouaha LR, Noguem B, Bakarnga-Via I, Nguepi MS, et al. Ethnopharmacological survey of Annonaceae medicinal plants used to treat malaria in four areas of Cameroon. J Ethnopharmacol. 2012;139:171–80. doi: 10.1016/j.jep.2011.10.035. [DOI] [PubMed] [Google Scholar]


