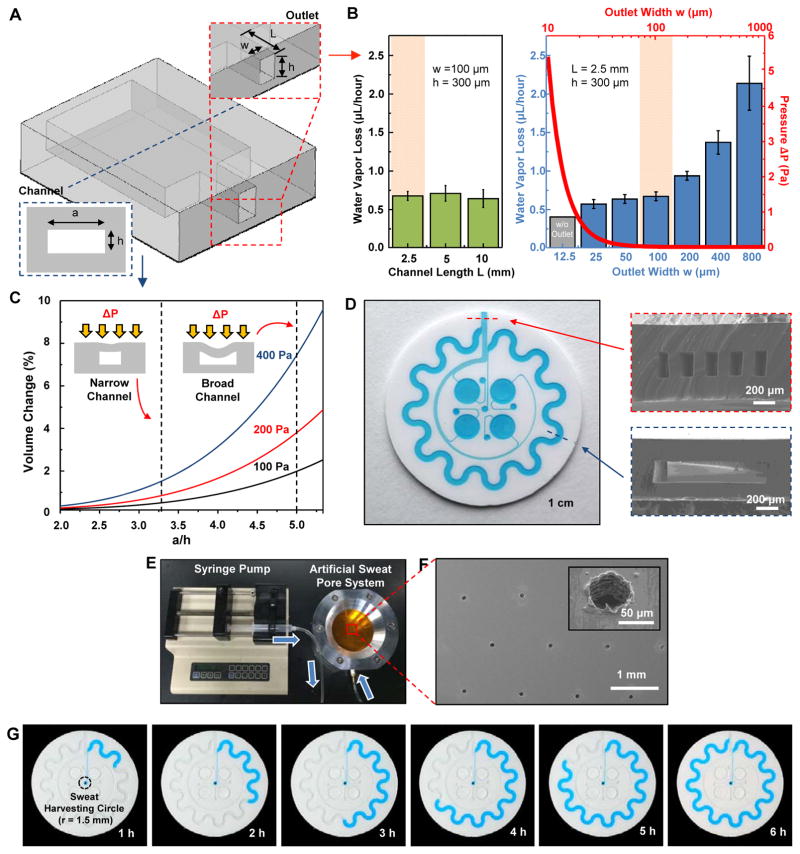
Fig. 2. Analysis of key design features and demonstration of epidermal microfluidic devices.
(A) Sketch of the channel geometry for numerical calculation. The blue and red dashed boxes highlight the dimensions of the serpentine and outlet channels, respectively. (B) Experimentally determined water vapor loss from a microfluidic channel as a function of width (w) and length (L) of the outlet channel with a fixed height of 300 μm. Inner pressure variation as a function of the outlet channel width was also determined from the model (red line). The orange shading highlights the optimal channel geometry. Data are presented as the average value, and error bars represent SD (n=3). (C) Model prediction of the change in volume of the serpentine channel as a function of AR [ratio of width a to height h of the serpentine channel in (A), blue dashed box] under various pressures (ΔP = 100, 200 and 400 Pa). ΔP represents pressure difference between the inside and outside of the serpentine channel. Dotted vertical lines show two representative ARs (10:3 and 5:1). (D) Picture of a fabricated epidermal microfluidic structure corresponding to the theoretical results and cross-sectional scanning electron microscope (SEM) images of the outlet (red dashed box) and serpentine (blue dashed box) channels. (E) Experimental setup of artificial sweat pore system. (F) SEM images of the polyimide (PI) membrane mimicking human sweat glands. (G) Demonstration of hydrodynamic fluid flow through the microfluidic device using the artificial sweat pore system at the rate of 5.5 μl/hour.