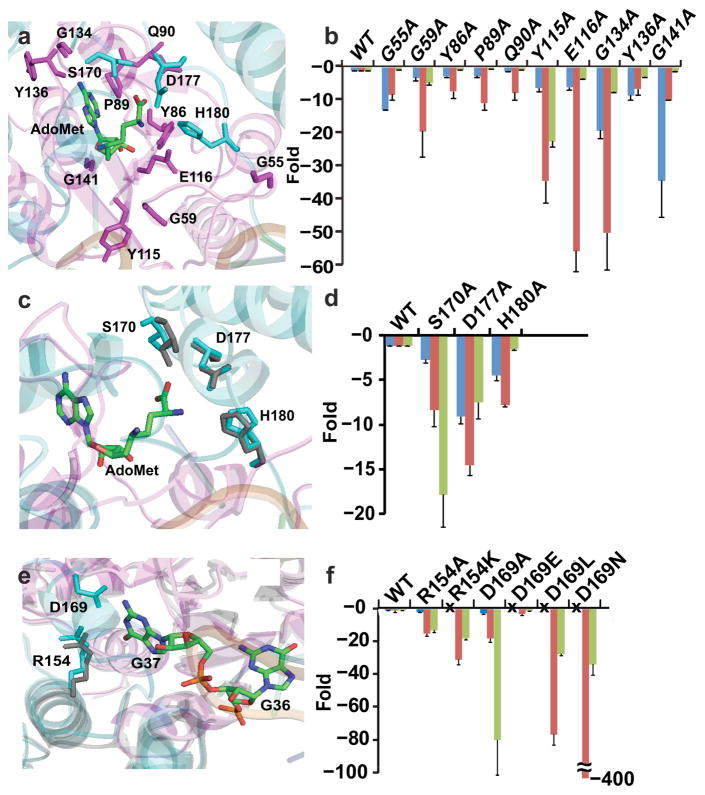
Figure 4.
Mutations leading to fold-changes in kinetic parameters of TrmD methyl transfer. Values from enzyme titration of tRNA were used for free energy analysis. Blue bars show fold-changes in Kd (AdoMet), red bars show fold-changes in Kd (tRNA), and green bars show fold-changes in kchem (methyl transfer). (a, b) Structures and residues tested by Ala substitutions of the Ado pocket (a) and the fold-change relative to the wild-type enzyme for each substitution (b). (c, d) Structures and residues tested by Ala substitutions of the Met pocket (c) and the fold-change relative to the wild-type enzyme for each substitution (d). (e, f) Structures and residues tested by Ala substitutions of the G37 pocket (e) and the fold-change relative to the wild-type enzyme for each substitution (f). The symbol “x” means not tested. Error bars represent s.d. (n = 5 independent measurements).