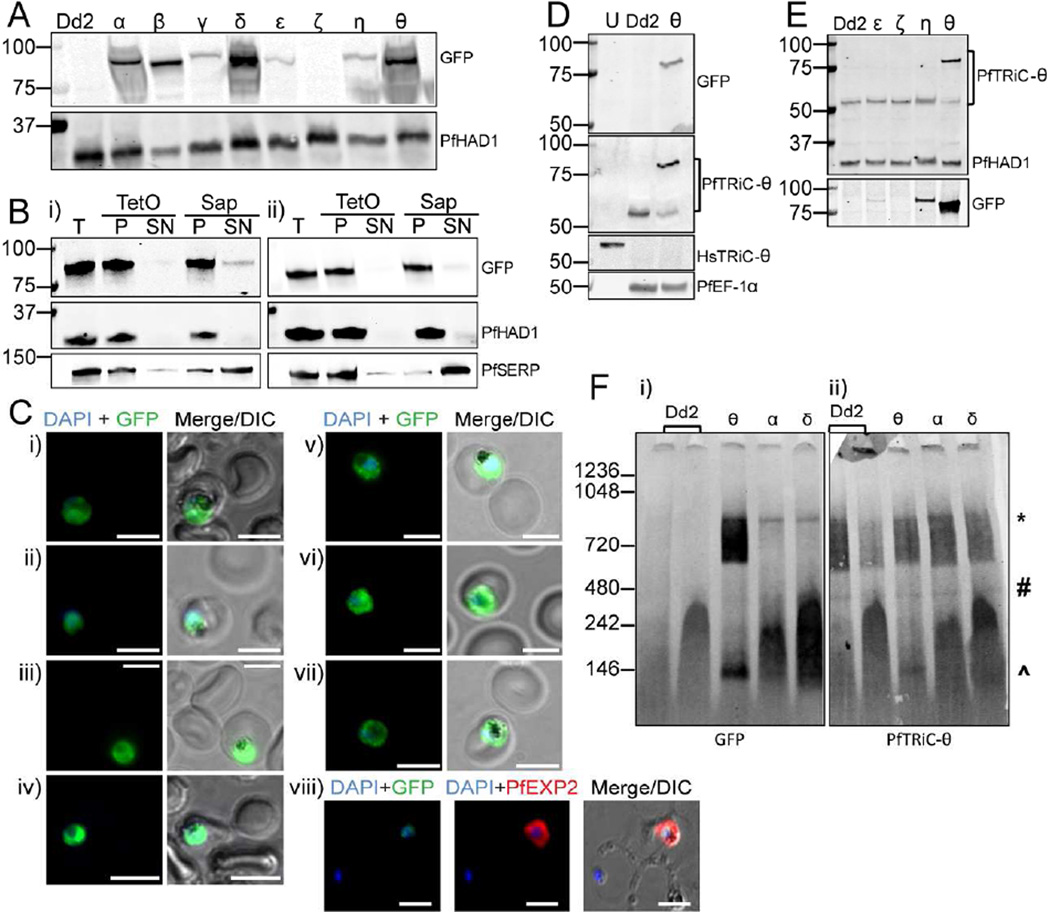
Figure 3. GFP-tagged PfTRiC subunits also form high molecular weight complexes in the parasite cytosolic fraction.
A, Ectopic expression of GFP-tagged PfTRiC subunits under the control of the strong, constitutive HSP86 promoter. Immunoblots show successful overexpression of all subunits, except PfTRiC-ζ. Dd2 is the parent parasite line and PfHAD1 is a loading control. B, Infected RBC samples (T = total sample) from the i) PfTRiC-η-GFP line and ii) PfTRiC-θ-GFP line were fractionated (SN = supernatant, P = pellet) with 0.05 % saponin (Sap; 20 s, room temperature) or 1 HU of TetO (20 min, 37 °C). PfSERP is an integrity marker for the parasitophorous vacuole and PfHAD1 is an integrity marker for the parasite compartment. C, IFA of paraformaldehyde-fixed RBC infected with PfTRiC-GFP lines i) α, ii) β, iii) γ, iv) δ, v) ε, vi) η and vii) θ. IFA was also performed on acetone-fixed RBC infected with PfTRiC-θ-GFP parasites probed with anti -PfTRiC-θ, and PfEXP2 to delineate the parasitophorous vacuole (viii). DAPI stains the parasite nuclei. Scale bars = 5 µm. Data are representative of two independent assays. D, Lysates from uninfected RBC (U), saponin-isolated trophozoite-stage infected RBC (Dd2; parent) or saponin-isolated PfTRiC-θ-GFP-infected RBC were probed with GFP or PfTRiC-θ antibodies. Parasite EF-1α is a parasite control marker, and HsTRiC-θ is the RBC control marker. E, Lysates from saponin-isolated PfTRiC-GFP parasites, and Dd2 parent parasites, probed with PfTRiC-θ antibody. GFP and PfHAD1 are loading controls. F, Saponin-isolated parasites (PfTRiC-α,δ and θ-GFP) were digitonin permeabilized, the soluble fraction was separated by blue-native polyacrylamide gel electrophoresis and the blot was probed with anti-GFP and anti-PfTRiC-θ. * = expected size of the heterohexadecamer. # = expected size of heterooctamer. ^ = expected size of monomers. Immunoblots are representative of three independent assays. Equivalent fractions were loaded in all blots. Marker bands are in kDa.