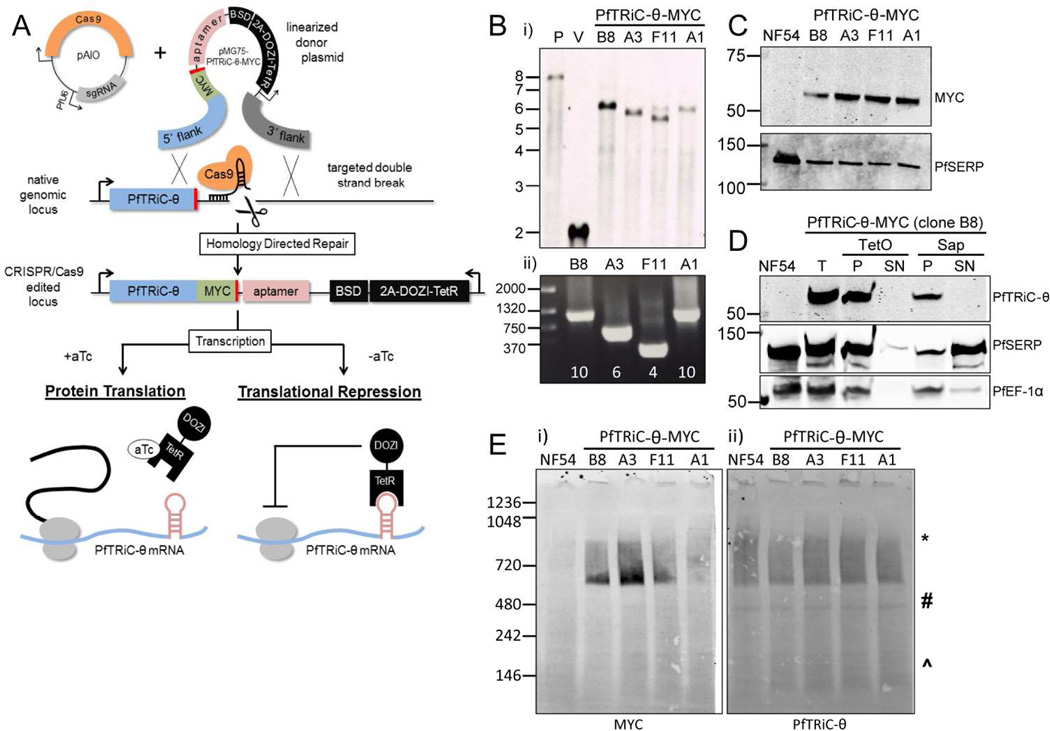
Figure 4. Generation of PfTRiC-θ-MYC aptamer-tagged lines.
A, Schematic outlining the strategy for tagging the 3’ end of PfTRiC-θ with a MYC epitope tag, and introducing an exogenous 3’ UTR containing an aptamer array. The desired integration event was achieved using CRISPR/Cas9 editing, using an “All-In-One” vector (pAIO) expressing both the sgRNA and Cas9 endonuclease. When anhydrotetracycline (aTc) is present it binds the Tet repressor (TetR), allowing translation of the PfTRiC-θ mRNA. When aTc is removed, the TetR binds the aptamer element in the 3’UTR, and the DOZI modifier targets the mRNA for sequestration/degradation, preventing translation of the PfTRiC-θ protein. B, i) Southern blot of digested vector (V) and genomic DNA from the NF54 parental line (P) and four tagged clones (probed with the 5’ homologous flank DNA), showing the expected integration event. Expected sizes are 7.9 kb for the parent, 2.0 kb for the vector and 6.0 kb for the integrants. Marker bands are in kb. ii) PRC screening using primers flanking the aptamer array, indicating the presence of a 10-element array in clones B8 and A1, 6-element array in clone A3 and 4-element array in F11. Marker bands are in bp. C, Lysates from NF54 and four PfTRiC-θ-MYC-tagged clones were prepared in RIPA buffer and immunoblots probed with anti-MYC antibody. PfSERP is a loading control. Immunoblots are representative of two independent experiments. Marker bands are in kDa. D, Infected RBC samples (T = total sample) from the PfTRiC-θ-MYC clone B8 parasite line were fractionated (SN = supernatant, P = pellet) with 0.05 % saponin (Sap; 20 s, room temperature) or 1 HU of TetO (20 min, 37 °C). PfSERP and PfEF1- α are integrity markers for the PV and parasite compartments, respectively. E, Saponin-isolated PfTRiC-θ-MYC clones were permeabilized with digitonin, the soluble fraction was separated by blue-native polyacrylamide gel electrophoresis and the blot was probed with anti-MYC and anti-PfTRiC-θ. * = expected size of the heterohexadecamer. # = expected size of heterooctamer. ^ = expected size of monomers. Equivalent fractions were loaded in all blots. Immunoblots are representative of two independent assays. Marker bands are in kDa.