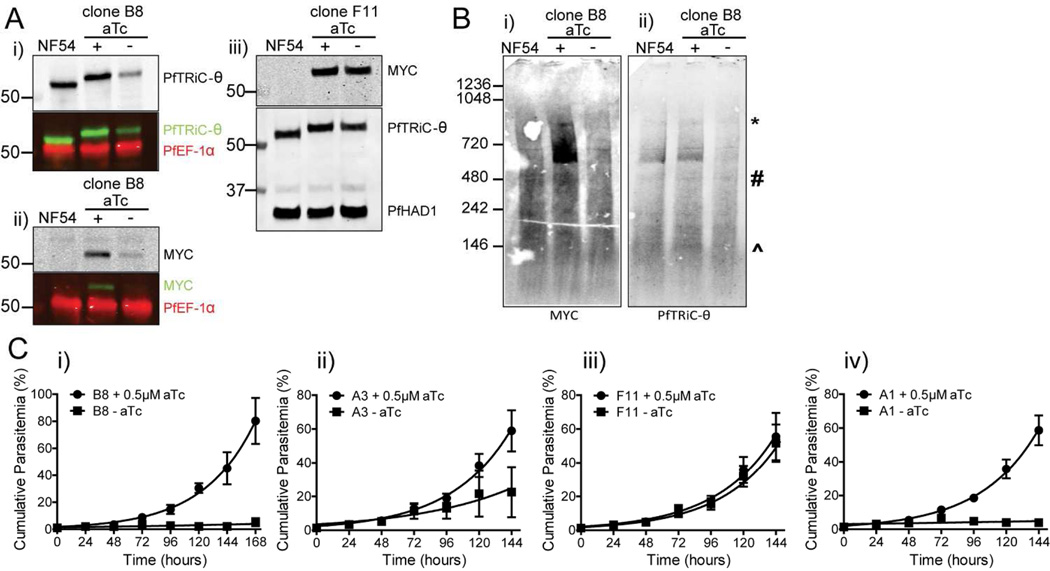
Figure 5. PfTRiC-θ is essential for parasite growth, and loss of the TRiC-θ subunit results in loss of the high molecular weight complex.
A, Lysates from NF54 and PfTRiC-θ-MYC-tagged clone B8, grown ± 0.5 µM aTc for 24 h, were prepared in RIPA buffer and immunoblots probed with i) anti- PfTRiC-θ antibody or ii) anti-MYC antibody. PfEF-1α is a loading control. In iii), lysates were prepared from the PfTRiC-θ-MYC-tagged clone F11 line. PfHAD1 is a loading control. Cell number equivalent fractions were loaded in all blots. Immunoblots are representative of four independent experiments. Marker bands are in kDa. B, Saponin-isolated NF54, or PfTRiC-θ-MYC clone B8, grown ± 0.5 µM aTc for 24 h, were digitonin permeabilized, the soluble fraction was separated by blue-native polyacrylamide gel electrophoresis and the blot was probed with i) anti-MYC and ii) anti-PfTRiC-θ. * = expected size of the heterohexadecamer. # = expected size of heterooctamer. ^ = expected size of monomers. Immunoblots are representative of two independent assays, with standard SDS-PAGE run in parallel (as in A) to confirm knockdown was achieved, and ensure equal parasite loading. Cell number equivalent fractions were loaded in all blots. Marker bands are in kDa. C, Growth assay, beginning with asynchronous parasite culture, of the four PfTRiC-θ-MYC-tagged clones, grown ± 0.5 µM aTc. Values are mean parasitemia, with error bars representing standard error of the mean from two independent experiments. The fitted line is an exponential growth equation (GraphPad Prism version 5).