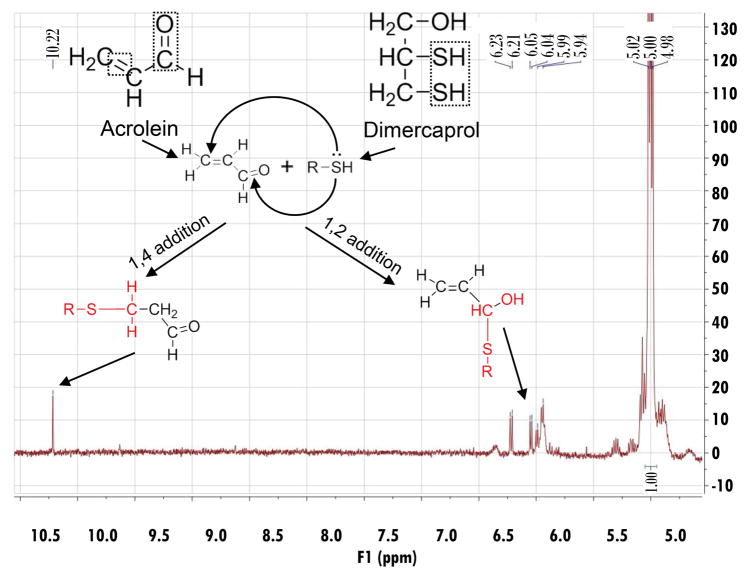
Figure 3.
The 1H-NMR spectrum of acrolein-dimercaprol reaction products. After recorded pure acrolein 1H-NMR spectrum in DMSO-d6, a solution of dimercaprol (50 mg) in DMSO-d6 (1 mL) was added. The mixture was incubated for 1 hr in the dark. The spectrum was obtained with a 300 MHz Bruker NMR spectrometer. The possible reaction mechanisms between dimercaprol and acrolein, and the structures of possible products were shown. In this assay, the dimercaprol was used in excess (final concentration was approximately 200 mM) compared to acrolein (final concentration was approximately 3.74 mM). This was designed to produce adequate acrolein-dimercaprol adducts to achieve measureable signals using NMR. The characteristic peaks for the aldehyde proton of acrolein were shifted downfield (δ 10.22), which indicates the formation of another new aldehyde. The peaks for the alkene protons of acrolein were shifted upfield (δ 6.23–5.94), which indicates the formation of another new alkene.