Figure 2.
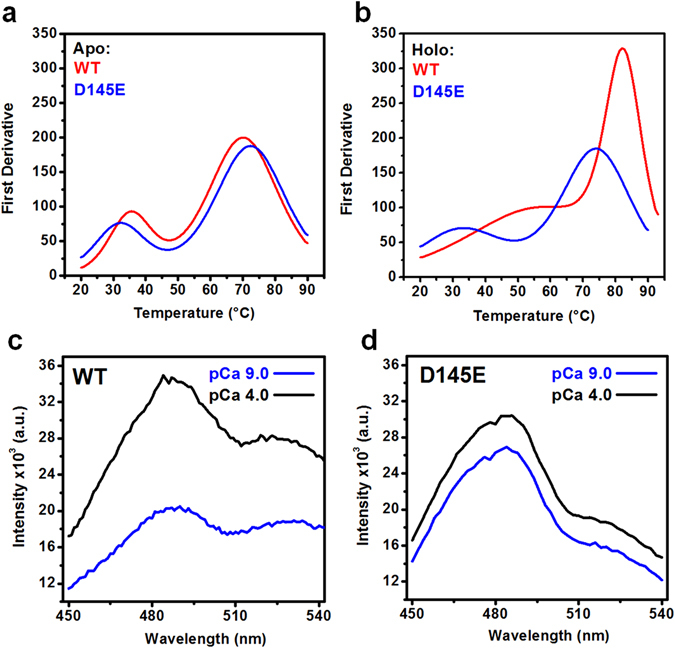
Thermostability and hydrophobic exposure of WT and D145E. Mean residue ellipticity at 222 nm was measured for HcTnC WT and D145E. Data shown are sample derivatives from 3 experiments with 2 different protein batches in apo (a) and Mg2+/Ca2+-bound (b) states. All melting curves were run in reverse after reaching 90 °C, and the structural changes promoted by temperature were reversible. For average values, see text. In (c), 5 μM bis-ANS was excited at 360 nm in the presence of 1 μM WT or D145E to compare fluorescence intensity at low (pCa 9.0) and high (pCa 4.0) Ca2+ concentrations as a measure of binding to hydrophobic surface areas. Average ratios (holo/apo ± s.e.m.) were 1.68 ± 0.11 for WT and 1.13 ± 0.02 for D145E (p = 0.007).
