Figure 3.
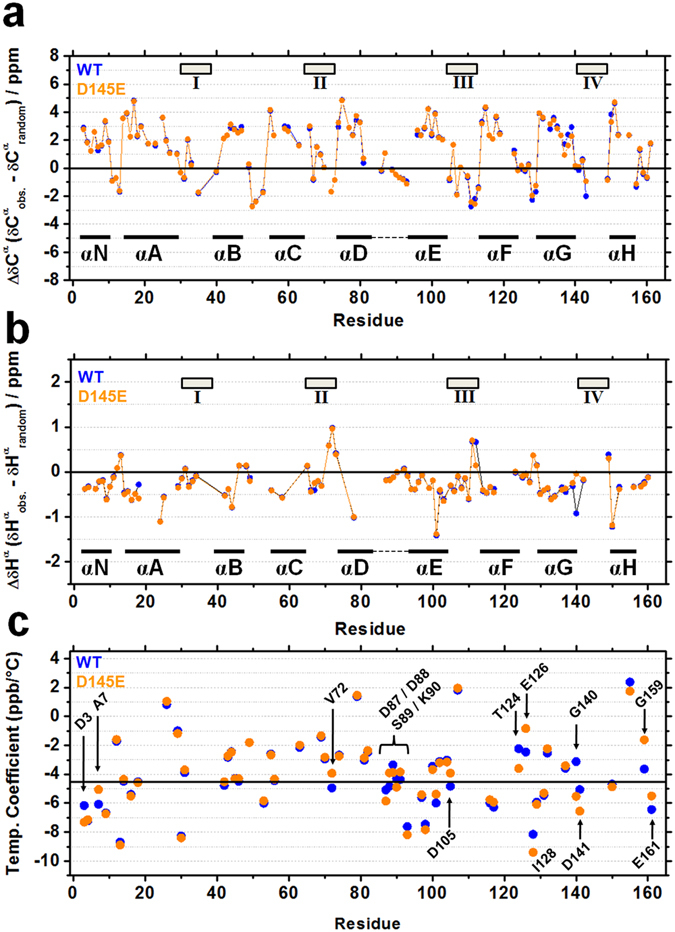
Secondary structure at 25 °C (a,b) and distribution of amide hydrogen temperature coefficients over the range 15°–55 °C (c) among WT ( ) and D145E (
) and D145E ( ) residues. Deviation of experimental (obs.) chemical shifts (δ) from random coils for backbone Cα (a) and Hα (b). Random-coil chemical shift values were obtained from ref. 27. Rectangles (I–IV) and black line segments (αN, αA, etc) identify Ca2+-binding sites and α-helices known to be present in WT cTnC. Alpha-helical segments show positive deviations for Cα and negative deviations for Hα, while β-strands and loops show the opposite. Dashed lines between α-D and α-E in a and b represent the D/E linker region. In (c), amide hydrogens with temperature coefficients that were linear but very different (>95% C.I.) for WT and mutant proteins are identified by residue. Temperature coefficients of the protons coincide (or differ only slightly) for the two isoforms except where one of the paired values is labeled with an arrow.
) residues. Deviation of experimental (obs.) chemical shifts (δ) from random coils for backbone Cα (a) and Hα (b). Random-coil chemical shift values were obtained from ref. 27. Rectangles (I–IV) and black line segments (αN, αA, etc) identify Ca2+-binding sites and α-helices known to be present in WT cTnC. Alpha-helical segments show positive deviations for Cα and negative deviations for Hα, while β-strands and loops show the opposite. Dashed lines between α-D and α-E in a and b represent the D/E linker region. In (c), amide hydrogens with temperature coefficients that were linear but very different (>95% C.I.) for WT and mutant proteins are identified by residue. Temperature coefficients of the protons coincide (or differ only slightly) for the two isoforms except where one of the paired values is labeled with an arrow.
