Figure 5.
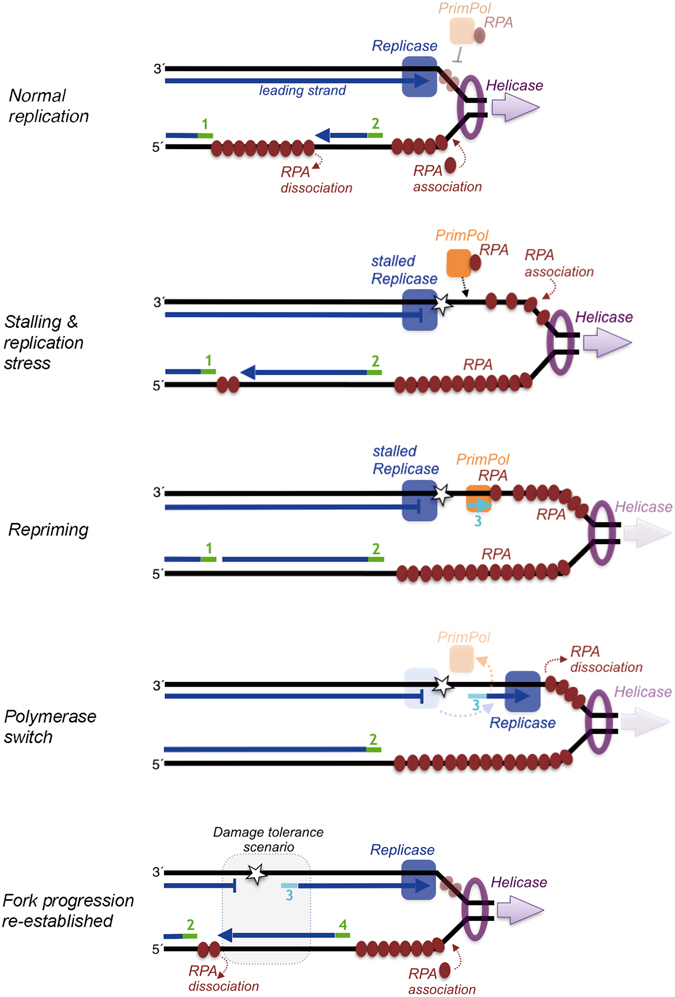
Model for PrimPol and RPA interactions at the replication fork during replicative stress. During normal replication fork progression, the leading replicase and the helicase are coordinated, RPA mainly binds to the lagging strand and PrimPol (depicted in association to RPA) has no access to the short ssDNA ahead of the leading replicase (likely covered by RPA). Under replicative stress, the leading strand replicase is stalled, and PrimPol/RPA now can gain access to the long stretches of ssDNA accumulated as a consequence of a sustained helicase unwinding, in a way compatible with an improved binding of RPA to ssDNA. PrimPol repriming in the leading strand triggers a polymerase switch that mobilizes the leading strand replicase from the stalling site to the new DNA primer, that becomes elongated, and the excess of RPA becoming displaced and dissociated. Recovery of the coordination between replicase and helicase re-establishes fork progression and normal lagging strand synthesis. The gap left behind in the leading strand constitutes a damage tolerance scenario, now accessible to translesion and/or repair machineries. For simplicity, the primase and polymerases acting on the lagging strand are not represented. RNA primers in the lagging strand (green) or an eventual DNA primer made by PrimPol in the leading strand (cyan) are numbered according to their proposed order of synthesis.
