Abstract
Ocean warming is driving species poleward, causing a ‘tropicalization’ of temperate ecosystems around the world. Increasing abundances of tropical herbivores on temperate reefs could accelerate declines in habitat-forming seaweeds with devastating consequences for these important marine ecosystems. Here we document an expansion of rabbitfish (Siganus fuscescens), a tropical herbivore, on temperate reefs in Western Australia following a marine heatwave and demonstrate their impact on local kelp forests (Ecklonia radiata). Before the heatwave there were no rabbitfish and low rates of kelp herbivory but after the heatwave rabbitfish were common at most reefs and consumption of kelp was high. Herbivory increased 30-fold and kelp abundance decreased by 70% at reefs where rabbitfish had established. In contrast, where rabbitfish were absent, kelp abundance and herbivory did not change. Video-analysis confirmed that rabbitfish were the main consumers of kelp, followed by silver drummers (Kyphosus sydneyanus), a temperate herbivore. These results represent a likely indirect effect of the heatwave beyond its acute impacts, and they provide evidence that range-shifting tropical herbivores can contribute to declines in habitat-forming seaweeds within a few years of their establishment.
Introduction
A general poleward shift in distribution of marine species due to ocean warming was forecast decades ago1–4. Since then, numerous studies have documented unusual and increasing occurrences of warm-water species of diverse phyla at higher latitudes5–8, such as hermatypic corals9, echinoderms10, 11, mollusks7 and fish11–14. Range expansions have been accentuated in regions where intensifying warm currents from the tropics accelerate warming at temperate latitudes11, 15. Such currents have increased the arrival of warm-water vagrant species and allowed them to remain at higher latitudes for longer periods of time, attain higher abundances and sometimes establish permanent populations11, 16. As a consequence, the proportion of tropical species increase, resulting in a ‘tropicalization’ of the community. In some ecosystems this change in species composition has led to increased competition and species displacement17, 18, modification of food-webs and ecosystem functioning19, and regime shifts caused by the arrival of habitat-modifying species, such as herbivorous urchins or fish8, 20.
Increasing abundances of tropical herbivorous fish on temperate reefs will prompt major changes and threaten the services that these ecosystems provide11. Temperate reefs are characterised by high abundances of habitat-forming seaweed, typically dominated by kelp21, which support high levels of biodiversity and multiple fishery resources22. Rates of herbivory by fish are generally low in temperate reef systems23–26, although in some places a few fish species have shown high-intensity but small-scale effects on seaweed communities27–30. In contrast, herbivorous fish play a critical role in healthy tropical reef ecosystems where they often facilitate corals by maintaining low abundances of seaweeds31; herbivorous fish typically attain higher abundances and greater diversity of species and feeding modes in tropical reefs in comparison to temperate reefs32–34. Increasing abundances of herbivorous fish on temperate reefs can translate into higher rates of herbivory and a reduced abundance of seaweed through direct consumption35 or indirectly by affecting seaweed reproduction36 and recruitment14. This can have cascading effects that encompass multiple trophic levels, and reduce the resistance and resilience of kelp ecosystems11, 14, 37. Moreover, a loss or reduction in habitat-forming seaweeds can also indirectly affect other systems, such as seagrass meadows, that often receive energy and nutrients from detached seaweeds from adjacent reefs38, 39.
Evidence for the impact of tropical herbivorous fish in temperate marine ecosystems has been increasing in recent years11, 40. Tropical parrotfish, which consume seagrass at higher rates than local temperate herbivores, are increasing in abundance in the northern Gulf of Mexico41. In southern Japan, temperate reefs now host permanent populations of tropical rabbitfish and parrotfish13 which have contributed to the decline of extensive kelp forests42–44. Eastern Mediterranean habitats that used to be dominated by seaweeds have been transformed into barren seascapes after the intrusion and settlement of tropical rabbitfish45, 46 through the Suez Canal47. South-eastern Australia has experienced an increase of tropical surgeonfishes48 and rabbitfishes35 that have caused the decline of kelp forests on offshore reefs35.
In Western Australia, an exceptional marine heatwave during the summer of 2010–201149–51 allowed the poleward migration and population expansion of the tropical rabbitfish Siganus fuscescens in the region14, 52, 53. This species is a characteristic herbivore of tropical reefs54–56, but now is an important consumer of kelp in south-eastern Australia35. Here we investigate if the heatwave, by allowing the expansion of rabbitfish, had indirect effects on kelp forests at latitudes lower than those at which it directly caused physiological impacts on kelp. We present a temporal analysis of changes in abundance of herbivorous fish, kelp and rates of herbivory from years prior to the marine heatwave (2004 and 2007) to the present (2016).
Results
Within the fish community, three species were identified as documented consumers of kelp: the temperate Kyphosus sydneyanus (silver drummer) and Olisthops cyanomelas (herring cale) and the tropical Siganus fuscescens (rabbitfish, Supplementary Table S1). Among these herbivores, K. sydneyanus was the most abundant species in 2004 and 2007 (2.2 ± 1.9 and 3.0 ± 2.6 individuals 125 m−2 [mean ± SE], respectively), followed by O. cyanomelas (0.3 ± 0.2 and 1.5 ± 0.7 individuals 125 m−2). However, abundances varied among reefs (PERMANOVA, pseudo-F 6, 51 = 2.84, P = 0.013). Cow Rocks had the highest abundance of temperate herbivorous fish and The Lumps had the lowest, while Whitfords Rock and Wreck Rock hosted intermediate abundances. S. fuscescens was not recorded on any reefs in 2004 or 2007 (Fig. 1a). In contrast, S. fuscescens was the most abundant herbivore recorded in 2016 (9.4 ± 2.1 individuals 125 m−2; Fig. 1a), representing 19% of all individual fish recorded. The abundance of K. sydneyanus (4.7 ± 2.7 individuals 125 m−2) and O. cyanomelas (0.2 ± 0.1 individuals 125 m−2) did not change between years and consistently represented ~10% of the total fish abundances recorded (PERMANOVA, pseudo-F 2, 51 = 1.98, P = 0.12). S. fuscescens was not recorded at The Lumps, which still had the lowest herbivore abundances; however, S. fuscescens was present on all other reefs, where Cow Rocks consistently had the highest abundances of herbivorous fish (Fig. 1a).
Figure 1.
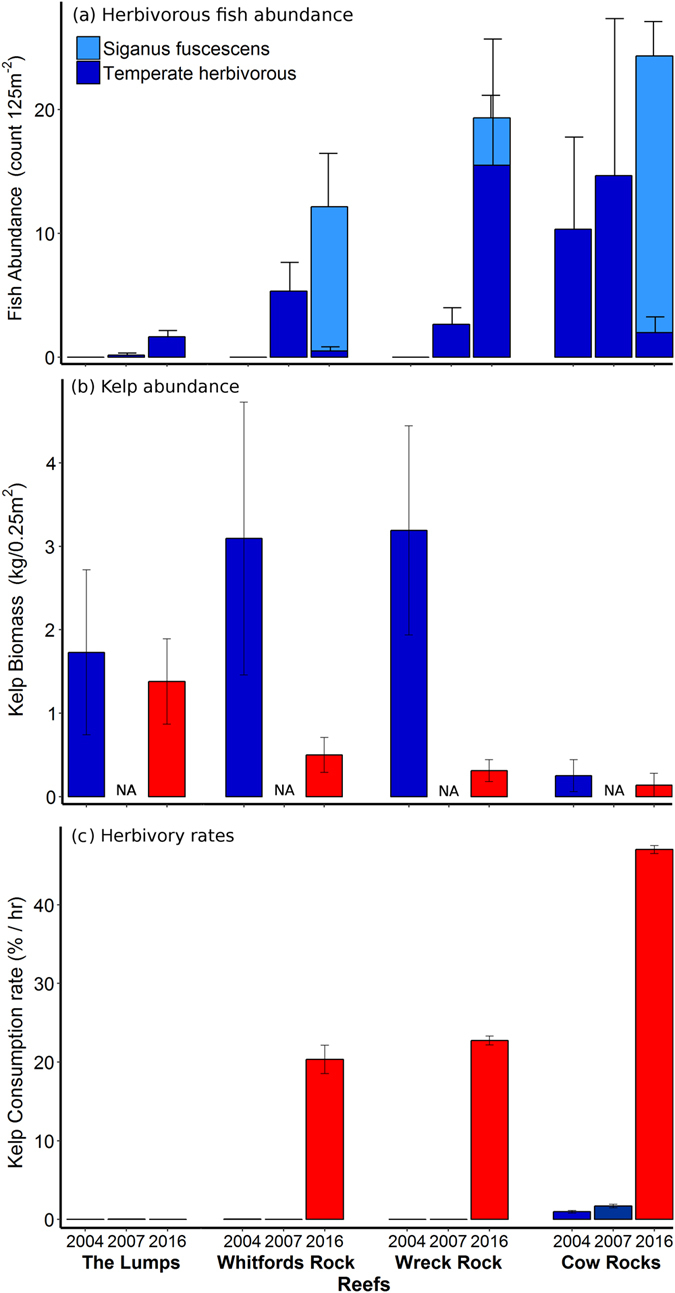
The abundance (mean ± SE) of herbivorous fish (temperate: Kyphosus sydneyanus and Olisthops cyanomelas) known to consume kelp (Ecklonia radiata) (a), kelp biomass (b) and herbivory rates on kelp (c) at temperate reefs of Marmion Marine Park, south-western Australia, from years before (2004, 2007) and after (2016) the marine heatwave of 2011.
Kelp biomass decreased from 2.1 ± 0.6 kg 0.25 m−2 in 2004 to 0.58 ± 0.17 kg 0.25 m−2 in 2016, representing a loss of 1.48 kg 0.25 m−2 or 71% (Fig. 1b; PERMANOVA, pseudo-F 1, 32 = 6.29, P = 0.017). Kelp declined most at Wreck Rock (84%) and Whitfords Rock (67%). At Cow Rocks, kelp abundance was low in both 2004 and 2016 (but still declined 10%), whereas at The Lumps kelp biomass remained high in both 2004 and 2016, showing no signs of decline (Fig. 1b).
Before the heatwave, there was no consumption of kelp tethers, except at Cow Rocks, where low herbivory rates were recorded (Fig. 1c). After the heatwave in 2016, kelp consumption rates were 30-fold higher than those recorded in 2004 and 2007 (Fig. 1c; PERMANOVA, pseudo-F 2, 78 = 3246, P = 0.0001). In 2016, 5-day herbivory assays resulted in 88.8% ± 2.6 consumption day−1 (mean ± SE; equivalent to ca. 3.7% hr−1) of kelp biomass across all reefs hosting rabbitfish. Caged kelp tethers did not show any loss of biomass, supporting the inference that kelp loss was due to consumption by fish. These rates far surpassed those measured over similar time periods prior to the heatwave (20.8% ± 0.1 day−1 in 2004 and 20.6% ± 0.1 day−1 in 2007; equivalent to ca. 0.9% hr−1). Additional 4 hr video-filmed herbivory assays in 2016 recorded consumption rates of 28.4% ± 2.9 hr−1 at reefs hosting rabbitfish, suggesting most of the consumption in the 2016 five-day assays occurred at higher rates than when calculated across the entire deployment time. In contrast to The Lumps where no herbivory and no rabbitfish were recorded (Fig. 1c), Cow Rocks had the highest herbivory rates (47.1% ± 0.5 hr−1), followed by Wreck Rock (22.7% ± 0.5 hr−1) and Whitfords Rock (20.3% ± 1.8 hr−1) (Fig. 1c; PERMANOVA, pseudo-F 6, 78 = 476, P = 0.001). Video-analyses of the herbivory assays confirmed that rabbitfish were responsible for all the consumption at Whitfords Rock and Wreck Rock, while consumption at Cow Rocks was shared between rabbitfish and silver drummers, which consumed approximately 50% each.
There was a significant positive relationship between rabbitfish abundance and herbivory rates (Fig. 2a; PERMANCOVA, pseudo-F 1, 8 = 98, P = 0.001); whilst there was no relationship between abundance of temperate herbivorous fish and herbivory rates (Fig. 2b; PERMANCOVA, pseudo-F 1, 6 = 0.1, P = 0.464). In turn, there was a significant negative relationship between kelp consumption rates on tethers and standing kelp biomass on the reefs (Fig. 2c; PERMANCOVA, pseudo-F 1, 4 = 11, P = 0.03).
Figure 2.
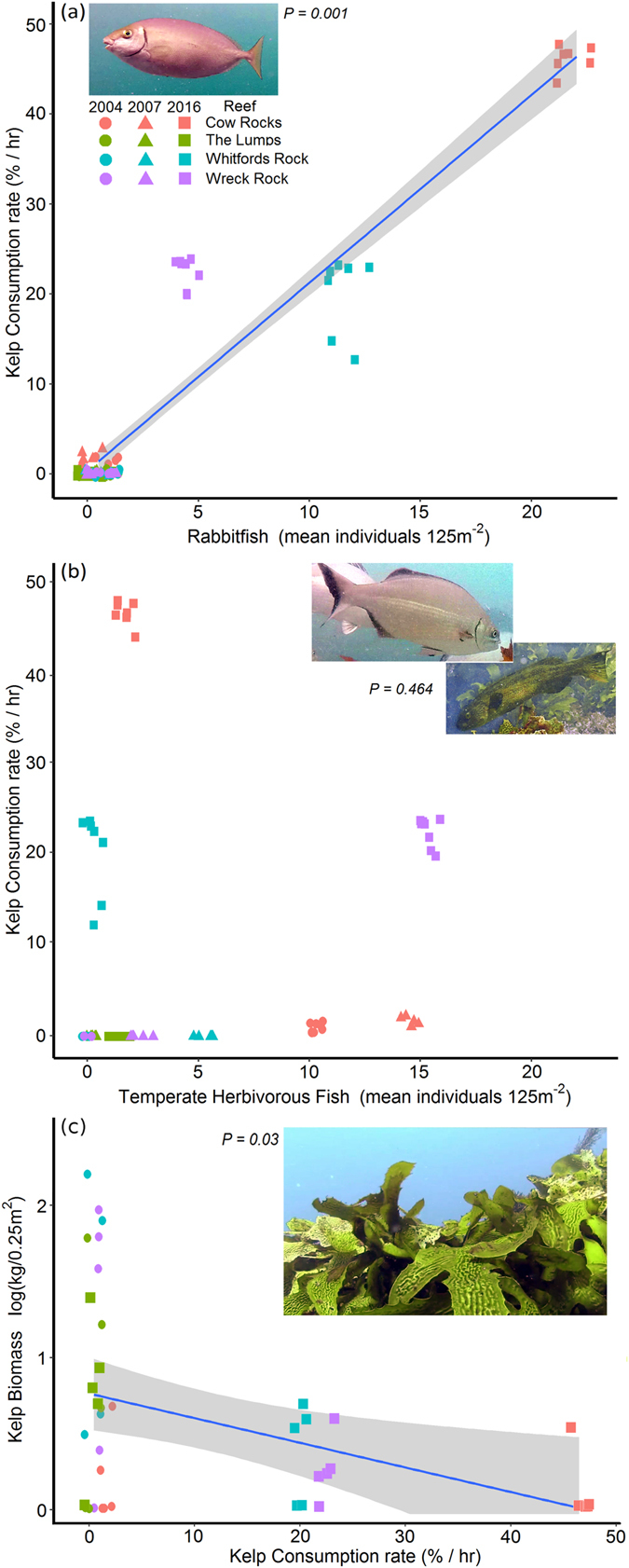
Relationship between herbivory rates and the abundance of rabbitfish (Siganus fuscescens), y = 1.33 + 2.05x, R2 = 0.89 (a), temperate herbivorous fish known to consume kelp (species pooled: Kyphosus sydneyanus and Olisthops cyanomelas), y = 5.88 + 0.407x, R2 = 0.02 (b), and kelp biomass (Ecklonia radiata), y = 1.85 − 0.0458x, R2 = 0.16 (c), on inshore reefs at Marmion, south-western Australia. Most of the points in (a) from before the marine heatwave (2004 and 2007) are clustered and overlapped close to zero. Regression line shown for statistically significant relationships, grey-shaded areas represent 95% confidence intervals and p-values were generated with PERMANCOVA.
Discussion
Our results document the establishment of rabbitfish (Siganus fuscescens) on temperate reefs off Perth following the 2011 marine heatwave, and show a concurrent steep increase in consumption rates of kelp. The abundance and rates of herbivory by two temperate herbivorous fishes (Kyphosus sydenayus and Olisthops cyanomelas) did not change among surveyed years, indicating that the increase in the consumption rates of kelp, and decrease in kelp abundance, is likely linked to the colonizing tropical rabbitfish — an inference that was supported by the video-analyses. Rabbitfish have not displaced the temperate herbivorous fish, but rather they have been an addition to the guild. Kyphosus sydneyanus consumed kelp at higher rates per individual than S. fuscescens, but kelp consumption by rabbitfish was higher due to their higher abundances. Siganus fuscescens has become now the most important kelp consumer on these reefs.
The broader spatial distribution of rabbitfish in south-western Australia after the heatwave is currently unknown, but we would expect to find similar patterns of abundance and herbivory across the other extensive inshore reefs in the region. The majority of the evidence suggest that S. fuscescens tends to inhabit shallow near-shore reefs adjacent to seagrass meadows. Past studies have found greater abundance closer to the coast53, 57. Although in south-eastern Australia rabbitfish were more common on offshore reefs, their abundance was low (2.2 ± 1.8 individuals 125 m−2)35. Our results suggest that S. fuscescens have specific habitat preferences. Despite being <1 km away from reefs hosting abundant rabbitfish populations they were absent at The Lumps, which is a slightly deeper (~7 m vs ~5 m) more contiguous reef environment than the other reefs surveyed. This may be related to the behaviour of tropical herbivorous fish which tend to prefer habitats with lower seaweed cover58. Further research is required to clarify these habitat affinities to make accurate predictions of future geographic distribution changes and associated impacts with habitat-forming seaweeds.
Past studies on the tropicalization of herbivore communities have regarded rabbitfish, including S. fuscescens, mainly as grazers14, 45. However, we found that S. fuscescens can also be an important browser in temperate ecosystems. This is in agreement with studies from south-eastern Australia35 and tropical reefs (Great Barrier Reef) that found S. fuscescens as the main consumer of Sargassum 54–56. These differences between studies are not surprising. Although S. fuscescens, and herbivores in general, often feed selectively from the available food sources59, they usually feed on a diverse mix of primary producers60, allowing them to play different functional roles57. We did not measure grazing rates but rabbitfish were observed biting on the substrate and epiphytic algae on seagrass shoots.
This feeding multifunctionality has important implications for the potential impact that these tropical herbivores can have in temperate ecosystems. High rates of grazing by rabbitfish can reduce the recruitment of habitat-forming seaweeds by consuming seaweeds in early-life stages, as they do in tropical reefs to maintain a seaweed-free state37, 61. Past studies have concluded that this was the main impact of rabbitfish on temperate seaweed communities14, 45. However, high rates of browsing by a single species can reduce kelp biomass considerably62. Rabbitfish have inhabited south-western Australia for at least three years52 and based on our video-filmed herbivory assays they have likely been consuming kelp throughout this period. Our results suggest that the decline in the biomass of kelp on inshore reefs has occurred due to increased consumption by rabbitfish. Wherever we observed rabbitfish we found an associated increase in herbivory and a decrease in kelp biomass; where they were absent, the opposite pattern was found. In addition to the direct reduction in kelp biomass, high levels of browsing can diminish the reproductive output of seaweeds36; although this remains poorly understood. In the case of E. radiata in the region, it produces the highest number of zoospores between April and May63, the same time of the year when our tethered kelps were consumed at high rates. Remaining kelps on the reefs with rabbitfish showed clear signs of heavy pruning by browsers (personal observation, S. Zarco-Perello & T. Wernberg), suggesting that rabbitfish do consume, and reduce, kelp reproductive tissue, potentially causing a decline in the supply of zoospores and abundances of gametophytes that later would become kelp recruits64.
It is likely that other factors have acted in synergy with herbivory to cause the decline of kelp. Temperature and UV radiation have been increasing over the last decades in the region15, 65, 66 and both can affect survival, growth and recruitment of kelp67–71. The marine heatwave that impacted the region in 2011 could have caused some mortality72, but not at the levels observed in our results at the latitude of our study sites8. The slightly deeper environments at The Lumps, the only reef with consistently high abundance of kelp, could have been less affected by these physical factors, although the difference in depth is not likely to be substantial enough to attenuate high temperatures or UV radiation67, 70, pointing to the low herbivory rates and its historical higher levels of productivity as the cause of its higher kelp abundance24.
The present study provides further evidence of the consequences of the globally emerging process of tropicalization of temperate reefs11, 40. As the poleward-migration of marine species is becoming more accentuated due to global warming, novel biological interactions within17, 73 and between trophic levels are emerging19, 74. The alteration of species richness and abundance of consumers impact lower trophic levels, leading to changes in ecosystem functioning and structure modifications75. Our results show that extreme marine heatwaves not only can have immediate devastating direct effects on temperate reefs8, 72 but also can have longer-term indirect effects by boosting the immigration of tropical herbivores that increase herbivory rates and reduce the abundance of habitat-forming seaweeds. This contrasts with observations from other tropicalized regions of the world where the process has been more gradual, such as Japan42, 43, the Mediterranean Sea45 and south-eastern Australia35. With the ongoing ocean warming65, 76, further range expansion and population increase of S. fuscescens is expected. Together with other environmental disturbances, such as high temperatures, these tropical herbivores pose a growing threat to temperate reefs, increasing the likelihood of regime shifts to vegetation-free states and the associated loss of environmental services of great value to human societies from these ecosystems.
Methods
Location
The study was carried out at four reefs off Marmion (Perth) in south-western Australia (31°49.4 S, 115°44.0 E), where studies in 2004 and 2007 had assessed species composition and abundances of fish, rates of kelp consumption by fish, and kelp abundance24, 38: Cow Rocks, Wreck Rock, Whitfords Rock and The Lumps, which all have similar environmental characteristics on depth and wave exposure. These reefs are characteristic of the inshore limestone reefs that exist along the coast of temperate south-western Australia. Here, the kelp Ecklonia radiata and fucoids such as Sargassum spp. dominate the reef flora, while adjacent seagrass meadows are dominated by Posidonia sinuosa and Amphibolis spp.24, 38. Historically, herbivory on these reefs has been low and mainly attributed to sea urchins with fish playing a minor and localised role24, 38.
Species Composition and Abundance of Herbivorous Fish
Fish surveys were undertaken in April 200438 and 2007 (M. Vanderklift unpublished data), and repeated in 2016 (this study). In 2004 and 2007 surveys were done by Underwater Visual Census (UVC). In 2016 the surveys were done by Stereo-Diver Operated Video (Stereo-DOV) following standard procedures14, 77 in order to generate a permanent visual record. Results produced by these two techniques are comparable in temperate environments78. In all years and at each reef, three 25 × 5 m transects were sampled along the ecotone between reef and seagrass on each of two separate days to account for spatio-temporal variability of the fish populations. Stereo-DOV videos were analysed with EventMeasure (SeaGIS Pty Ltd) where all individual fishes were counted and identified to the lowest taxonomic level possible14. Fish known to be kelp consumers (Supplementary Table S1) were grouped as herbivorous and separated by climate affinity (i.e. tropical, subtropical, temperate) based on information published on FishBase. The abundances of these species were used in the analysis of changes in consumption rates on kelp.
Kelp Abundance and Rates of Herbivory
Kelp abundance was quantified in 2004 and 2016 by sampling standing biomass within five quadrats (0.25 m2) haphazardly located at each reef, harvesting all kelp and measuring its wet weight38.
Rates of kelp consumption were assessed from kelp tethering assays following the methods used in previous studies at the same reefs24, 38. At each reef, at least seven individual ~15 cm long lateral blades of E. radiata were attached at the top of a 0.5 meter rod fixed to the substratum to simulate kelp canopy. In addition, three caged control tethers were deployed at each reef to test for possible non-fish sources of kelp loss, so loss could be confidently attributed to fish herbivory24, 38. Kelp lateral blades were photographed at the beginning and at the end of each deployment to measure the consumed area per time (% loss t–1) using the software ImageJ (rsb.info.nih.gov/ij/)24. Kelp tethers were deployed for 5 days in all years24, 38 and, in addition, in 2016 4-hour deployments were run at each reef while filming the consumption with video-cameras (GoPro) to refine the rates of herbivory and identify the species responsible for the consumption of kelp14. A HOBO pendant logger recorded a mean temperature of 21 ± 0.01 °C during the 2016 deployments.
Statistical Analysis
We tested for differences in abundance of herbivorous fish, kelp and herbivory rates between 2016 and previous years with Permutational Multivariate Analysis of Variance (PERMANOVA79 using the software PRIMER 6 & PERMANOVA+ (PRIMER-E Ltd) because of its robustness against assumptions of normality and homogeneity of variances in the data. Changes in abundance of temperate herbivorous fishes known to be kelp consumers (Kyphosus sydneyanus and Olisthops cyanomelas) through time were tested with two-way PERMANOVA with planned contrasts between the years before the heatwave (2004 and 2007) and post-heatwave (2016) based on Euclidean distances calculated from square-root transformed abundance data. Changes of kelp abundance through time were tested with a two-way PERMANOVA between 2004 and 2016. Changes in herbivory rates through time were analyzed with a two-way PERMANOVA with planned contrasts as explained previously based on Euclidean distances. The effect of the abundance of herbivores on herbivory rates was analysed with a two-way PERMANCOVA (i.e. Permutational Multivariate Analysis of Covariance), considering rabbitfish (S. fuscescens) and temperate herbivorous fish abundances as explanatory covariates in independent tests, while the effect of herbivory rates on kelp biomass changes was tested with a two-way PERMANCOVA, considering herbivory rates as the explanatory covariate with Euclidean distance as the resemblance measure. All tests encompassed 9999 permutations and were performed under the same statistical design, with years and reefs as fixed factors.
Electronic supplementary material
Documented species of temperate/subtropical and tropical herbivorous fish that consume kelp in different parts of the world.
Acknowledgements
We are grateful for assistance in the field from C. Tuckett, R. Hemsworth, S. Dee and Y. Mulders, and for the help with the stereo video analyses from T. Bond and M. Birt. This work was funded by grants from the Australian Research Council (DP170100023) and The Hermon Slade Foundation (HSF13-13) to TW.
Author Contributions
Conceived the study: T.W., S.Z.P. Collected data: S.Z.P., M.V., T.W. Analysed data: S.Z.P., T.L. Wrote the paper: S.Z.P., T.W. with input from all authors. Provided funding: T.W.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-00991-2
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Salvador Zarco-Perello, Email: salvador.zarco.perello@gmail.com.
Thomas Wernberg, Email: thomas.wernberg@uwa.edu.au.
References
- 1.Kennedy VS. Anticipated Effects of Climate Change on Estuarine and Coastal Fisheries. Fisheries. 1990;15:16–24. doi: 10.1577/1548-8446(1990)015<0016:AEOCCO>2.0.CO;2. [DOI] [Google Scholar]
- 2.Tonn WM. Climate Change and Fish Communities: A Conceptual Framework. Trans. Am. Fish. Soc. 1990;119:337–352. doi: 10.1577/1548-8659(1990)119<0337:CCAFCA>2.3.CO;2. [DOI] [Google Scholar]
- 3.Southward AJ, Hawkins SJ, Burrows MT. Seventy years’ observations of changes in distribution and abundance of zooplankton and intertidal organisms in the western English Channel in relation to rising sea temperature. J. Therm. Biol. 1995;20:127–155. doi: 10.1016/0306-4565(94)00043-I. [DOI] [Google Scholar]
- 4.Holbrook SJ, Schmitt RJ, Stephens JS. Changes in an Assemblage of Temperate Reef Fishes Associated with a Climate Shift. Ecol. Appl. 1997;7:1299–1310. doi: 10.1890/1051-0761(1997)007[1299:CIAAOT]2.0.CO;2. [DOI] [Google Scholar]
- 5.Hawkins SJ, et al. Consequences of climate-driven biodiversity changes for ecosystem functioning of North European rocky shores. Mar. Ecol. Prog. Ser. 2009;396:245–259. doi: 10.3354/meps08378. [DOI] [Google Scholar]
- 6.Hoegh-Guldberg O, Bruno JF. The Impact of Climate Change on the World's Marine Ecosystems. Science. 2010;328:1523–1528. doi: 10.1126/science.1189930. [DOI] [PubMed] [Google Scholar]
- 7.Pitt NR, Poloczanska ES, Hobday AJ. Climate-driven range changes in Tasmanian intertidal fauna. Mar. Freshwater Res. 2010;61:963–970. doi: 10.1071/MF09225. [DOI] [Google Scholar]
- 8.Wernberg T, et al. Climate-driven regime shift of a temperate marine ecosystem. Science. 2016;353:169–172. doi: 10.1126/science.aad8745. [DOI] [PubMed] [Google Scholar]
- 9.Yamano H, Hiroya Y, Kaoru S, Keiichi N. Rapid poleward range expansion of tropical reef corals in response to rising sea surface temperatures. Geophys. Res. Lett. 2011;38:L04601–n/a. doi: 10.1029/2010GL046474. [DOI] [Google Scholar]
- 10.Ling SD. Range expansion of a habitat-modifying species leads to loss of taxonomic diversity: a new and impoverished reef state. Oecologia. 2008;156:883–894. doi: 10.1007/s00442-008-1043-9. [DOI] [PubMed] [Google Scholar]
- 11.Vergés A, et al. The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proc. Biol. Sci. 2014;281:20140846–20140846. doi: 10.1098/rspb.2014.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feary DA, et al. Latitudinal shifts in coral reef fishes: why some species do and others do not shift. Fish Fish. 2013;15:593–615. doi: 10.1111/faf.12036. [DOI] [Google Scholar]
- 13.Nakamura Y, Feary DA, Kanda M, Yamaoka K. Tropical fishes dominate temperate reef fish communities within western Japan. PLoS One. 2013;8:e81107. doi: 10.1371/journal.pone.0081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett S, et al. Tropical herbivores provide resilience to a climate-mediated phase shift on temperate reefs. Ecol. Lett. 2015;18:714–723. doi: 10.1111/ele.12450. [DOI] [PubMed] [Google Scholar]
- 15.Wu L, et al. Enhanced warming over the global subtropical western boundary currents. Nat. Clim. Chang. 2012;2:161–166. doi: 10.1038/nclimate1353. [DOI] [Google Scholar]
- 16.Figueira WF, Booth DJ. Increasing ocean temperatures allow tropical fishes to survive overwinter in temperate waters. Glob. Chang. Biol. 2010;16:506–516. doi: 10.1111/j.1365-2486.2009.01934.x. [DOI] [Google Scholar]
- 17.Milazzo M, Mirto S, Domenici P, Gristina M. Climate change exacerbates interspecific interactions in sympatric coastal fishes. J. Anim. Ecol. 2013;82:468–477. doi: 10.1111/j.1365-2656.2012.02034.x. [DOI] [PubMed] [Google Scholar]
- 18.Galaiduk R, Figueira WF, Kingsford MJ, Curley BG. Factors driving the biogeographic distribution of two temperate Australian damselfishes and ramifications for range shifts. Mar. Ecol. Prog. Ser. 2013;484:189–202. doi: 10.3354/meps10300. [DOI] [Google Scholar]
- 19.Albouy C, et al. From projected species distribution to food-web structure under climate change. Glob. Chang. Biol. 2014;20:730–741. doi: 10.1111/gcb.12467. [DOI] [PubMed] [Google Scholar]
- 20.Mollmann C, Folke C, Edwards M, Conversi A. Marine regime shifts around the globe: theory, drivers and impacts. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;370:20130260–20130260. doi: 10.1098/rstb.2013.0260. [DOI] [Google Scholar]
- 21.Schiel DR, Foster MS. The structure of subtidal algal stands in temperate waters. Oceanogr. Mar. Biol. Annu. Rev. 1986;24:265–307. [Google Scholar]
- 22.Bennett S, et al. The ‘Great Southern Reef’: social, ecological and economic value of Australia’s neglected kelp forests. Mar. Freshwater Res. 2016;67:47–56. doi: 10.1071/MF15232. [DOI] [Google Scholar]
- 23.Choat JH. Fish Feeding and the Structure of Benthic Communities in Temperate Waters. Annu. Rev. Ecol. Syst. 1982;13:423–449. doi: 10.1146/annurev.es.13.110182.002231. [DOI] [Google Scholar]
- 24.Vanderklift MA, Lavery PS, Waddington KI. Intensity of herbivory on kelp by fish and sea urchins differs between inshore and offshore reefs. Mar. Ecol. Prog. Ser. 2009;376:203–211. doi: 10.3354/meps07811. [DOI] [Google Scholar]
- 25.Mann, K. H. Ecology of Coastal Waters: With Implications For Management (John Wiley & Sons, 2009).
- 26.Poore AGB, et al. Global patterns in the impact of marine herbivores on benthic primary producers. Ecol. Lett. 2012;15:912–922. doi: 10.1111/j.1461-0248.2012.01804.x. [DOI] [PubMed] [Google Scholar]
- 27.Harris LG, Ebeling AW, Laur DR, Rowley RJ. Community recovery after storm damage: a case of facilitation in primary succession. Science. 1984;224:1336–1338. doi: 10.1126/science.224.4655.1336. [DOI] [PubMed] [Google Scholar]
- 28.Andrew NL, Jones GP. Patch formation by herbivorous fish in a temperate Australian kelp forest. Oecologia. 1990;85:57–68. doi: 10.1007/BF00317343. [DOI] [PubMed] [Google Scholar]
- 29.Sala E, Boudouresque CF. The role of fishes in the organization of a Mediterranean sublittoral community. J. Exp. Mar. Bio. Ecol. 1997;212:25–44. doi: 10.1016/S0022-0981(96)02745-1. [DOI] [Google Scholar]
- 30.Taylor DI, Schiel DR. Algal populations controlled by fish herbivory across a wave exposure gradient on southern temperate shores. Ecology. 2010;91:201–211. doi: 10.1890/08-1512.1. [DOI] [PubMed] [Google Scholar]
- 31.Hughes TP, et al. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr. Biol. 2007;17:360–365. doi: 10.1016/j.cub.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 32.Meekan MG, Choat JH. Latitudinal variation in abundance of herbivorous fishes: a comparison of temperate and tropical reefs. Mar. Biol. 1997;128:373–383. doi: 10.1007/s002270050103. [DOI] [Google Scholar]
- 33.Floeter SR, Ferreira CEL, Dominici-Arosemena A, Zalmon IR. Latitudinal gradients in Atlantic reef fish communities: trophic structure and spatial use patterns. J. Fish Biol. 2004;64:1680–1699. doi: 10.1111/j.0022-1112.2004.00428.x. [DOI] [Google Scholar]
- 34.Bennett S, Bellwood DR. Latitudinal variation in macroalgal consumption by fishes on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2011;426:241–252. doi: 10.3354/meps09016. [DOI] [Google Scholar]
- 35.Vergés A, et al. Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. Proc. Natl. Acad. Sci. USA. 2016;133:13791–13796. doi: 10.1073/pnas.1610725113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nordemar I, Sjöö GL, Mörk E, McClanahan TR. Effects of estimated herbivory on the reproductive potential of four East African algal species – a mechanism behind ecosystem shifts on coral reefs? Hydrobiologia. 2007;575:57–68. doi: 10.1007/s10750-006-0282-1. [DOI] [Google Scholar]
- 37.Bellwood DR, Hughes TP, Folke C, Nyström M. Confronting the coral reef crisis. Nature. 2004;429:827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- 38.Wernberg T, Vanderklift MA, How J, Lavery PS. Export of detached macroalgae from reefs to adjacent seagrass beds. Oecologia. 2006;147:692–701. doi: 10.1007/s00442-005-0318-7. [DOI] [PubMed] [Google Scholar]
- 39.Bakker ES, et al. Herbivory on freshwater and marine macrophytes: A review and perspective. Aquat. Bot. 2016;135:18–36. doi: 10.1016/j.aquabot.2016.04.008. [DOI] [Google Scholar]
- 40.Hyndes GA, et al. accelerating tropicalization and the transformation of temperate seagrass meadows. Bioscience. 2016;66:938–948. doi: 10.1093/biosci/biw111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heck KL, Jr, Fodrie FJ, Madsen S, Baillie CJ, Byron DA. Seagrass consumption by native and a tropically associated fish species: potential impacts of the tropicalization of the northern Gulf of Mexico. Mar. Ecol. Prog. Ser. 2015;520:165–173. doi: 10.3354/meps11104. [DOI] [Google Scholar]
- 42.Nimura K, Takatsuji H, Masuda S, Shimamoto J. Growth and maturation of Ecklonia cava and Eisenia arborea seedlings transplanted along the coast of Hainan, Shizuoka prefecture and the grazing caused by herbivorous fish Siganus fuscescens. Aquaculture Sci. 2007;55:541–546. [Google Scholar]
- 43.Yamaguchi, A., Furumitsu, K., Yagishita, N. & Kume, G. Biology of herbivorous fish in the coastal areas of western Japan in Coastal environmental and ecosystem issues of the East China Sea (eds. Ishimatsu, A. & Lie, H. J.) 181–190 (Terrapub, 2010).
- 44.Nagai, S., Yoshida, G., & Tarutani K. Change in species composition and distribution of algae in the coastal waters of western Japan in global warming impacts - case studies on the economy, human health, and on urban and natural environments (ed. Casalegno, S.) 209-236 (Intech, 2011).
- 45.Vergés A, et al. Tropical rabbitfish and the deforestation of a warming temperate sea. J. Ecol. 2014;102:1518–1527. doi: 10.1111/1365-2745.12324. [DOI] [Google Scholar]
- 46.Sala E, Kizilkaya Z, Yildirim D, Ballesteros E. Alien marine fishes deplete algal biomass in the Eastern Mediterranean. PLoS One. 2011;6:e17356. doi: 10.1371/journal.pone.0017356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bariche M, Michel B, Riyad S, Ernesto A. Fecundity and condition of successful invaders: Siganus rivulatus and S. luridus (Actinopterygii: Perciformes: Siganidae) in the eastern Mediterranean Sea. Acta Ichthyol. Pisc. 2009;39:11–18. doi: 10.3750/AIP2009.39.1.03. [DOI] [Google Scholar]
- 48.Basford AJ, et al. Feeding habits of range-shifting herbivores: tropical surgeonfishes in a temperate environment. Mar. Freshwater Res. 2016;67:75–83. doi: 10.1071/MF14208. [DOI] [Google Scholar]
- 49.Feng M, McPhaden MJ, Xie S-P, Hafner J. La Niña forces unprecedented Leeuwin Current warming in 2011. Sci. Rep. 2013;3:1277. doi: 10.1038/srep01277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pearce AF, Feng M. The rise and fall of the ‘marine heat wave’ off Western Australia during the summer of 2010/2011. J. Mar. Syst. 2013;111–112:139–156. doi: 10.1016/j.jmarsys.2012.10.009. [DOI] [Google Scholar]
- 51.Hobday AJ, et al. A hierarchical approach to defining marine heatwaves. Prog. Oceanogr. 2016;141:227–238. doi: 10.1016/j.pocean.2015.12.014. [DOI] [Google Scholar]
- 52.Caputi, N. et al. Management Implications of Climate Change Effect on Fisheries in Western Australia, Part 1: Environmental Change and Risk Assessment FRDC Project No. 2010/535. Fisheries Research Report 260 (Department of Fisheries of Western Australia, 2014).
- 53.Richards Z, et al. marine biodiversity in temperate western Australia: multi-taxon surveys of Minden and Roe reefs. Diversity. 2016;8:7. doi: 10.3390/d8020007. [DOI] [Google Scholar]
- 54.Mantyka CS, Bellwood DR. Direct evaluation of macroalgal removal by herbivorous coral reef fishes. Coral Reefs. 2007;26:435–442. doi: 10.1007/s00338-007-0214-1. [DOI] [Google Scholar]
- 55.Cvitanovic C, Bellwood DR. Local variation in herbivore feeding activity on an inshore reef of the Great Barrier Reef. Coral Reefs. 2008;28:127–133. doi: 10.1007/s00338-008-0433-0. [DOI] [Google Scholar]
- 56.Fox RJ, Bellwood DR. Remote video bioassays reveal the potential feeding impact of the rabbitfish Siganus canaliculatus (f: Siganidae) on an inner-shelf reef of the Great Barrier Reef. Coral Reefs. 2008;3:605–615. doi: 10.1007/s00338-008-0359-6. [DOI] [Google Scholar]
- 57.Hoey AS, Brandl SJ, Bellwood DR. Diet and cross-shelf distribution of rabbitfishes (f. Siganidae) on the northern Great Barrier Reef: implications for ecosystem function. Coral Reefs. 2013;32:973–984. doi: 10.1007/s00338-013-1043-z. [DOI] [Google Scholar]
- 58.Hoey AS, Bellwood DR. Suppression of herbivory by macroalgal density: a critical feedback on coral reefs? Ecol. Lett. 2011;14:267–273. doi: 10.1111/j.1461-0248.2010.01581.x. [DOI] [PubMed] [Google Scholar]
- 59.Pillans RD, Franklin CE, Tibbetts IR. Food choice in Siganus fuscescens: influence of macrophyte nutrient content and availability. J. Fish Biol. 2004;64:297–309. doi: 10.1111/j.0022-1112.2004.00261.x. [DOI] [Google Scholar]
- 60.Azzurro E, et al. Resource partitioning among early colonizing Siganus luridus and native herbivorous fish in the Mediterranean: an integrated study based on gut-content analysis and stable isotope signatures. J. Mar. Biol. Assoc. UK. 2007;87:991–998. doi: 10.1017/S0025315407056342. [DOI] [Google Scholar]
- 61.Mumby PJ, et al. Fishing, trophic cascades, and the process of grazing on coral reefs. Science. 2006;311:98–101. doi: 10.1126/science.1121129. [DOI] [PubMed] [Google Scholar]
- 62.Bellwood DR, Hughes TP, Hoey AS. Sleeping functional group drives coral-reef recovery. Curr. Biol. 2006;16:2434–2439. doi: 10.1016/j.cub.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 63.Mohring MB, Wernberg T, Kendrick GA, Rule MJ. Reproductive synchrony in a habitat-forming kelp and its relationship with environmental conditions. Mar. Biol. 2012;160:119–126. doi: 10.1007/s00227-012-2068-5. [DOI] [Google Scholar]
- 64.Steneck RS, Graham MH, Bourque BJ. Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ. Conserv. 2002;29:436–459. doi: 10.1017/S0376892902000322. [DOI] [Google Scholar]
- 65.Lough JM. Shifting climate zones for Australia's tropical marine ecosystems. Geophys. Res. Lett. 2008;35:L14708. doi: 10.1029/2008GL034634. [DOI] [Google Scholar]
- 66.Herman JR. Global increase in UV irradiance during the past 30 years (1979–2008) estimated from satellite data. J. Geophys. Res. 2010;115:D04203. [Google Scholar]
- 67.Wood WF. Effect of solar ultra-violet radiation on the kelp Ecklonia radiata. Mar. Biol. 1987;96:143–150. doi: 10.1007/BF00394848. [DOI] [Google Scholar]
- 68.Wernberg T, et al. Decreasing resilience of kelp beds along a latitudinal temperature gradient: potential implications for a warmer future. Ecol. Lett. 2010;13:685–694. doi: 10.1111/j.1461-0248.2010.01466.x. [DOI] [PubMed] [Google Scholar]
- 69.Xiao X, et al. Sensitivity and acclimation of three canopy-forming seaweeds to UVB radiation and warming. PLoS One. 2015;10:e0143031. doi: 10.1371/journal.pone.0143031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bearham D, Vanderklift MA, Gunson JR. Temperature and light explain spatial variation in growth and productivity of the kelp Ecklonia radiata. Mar. Ecol. Prog. Ser. 2013;476:59–70. doi: 10.3354/meps10148. [DOI] [Google Scholar]
- 71.Mohring MB, Kendrick GA, Wernberg T, Rule MJ, Vanderklift MA. Environmental influences on kelp performance across the reproductive period: an ecological trade-off between gametophyte survival and growth? PLoS One. 2013;8:e65310. doi: 10.1371/journal.pone.0065310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wernberg T, et al. An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nat. Clim. Chang. 2013;3:78–82. doi: 10.1038/nclimate1627. [DOI] [Google Scholar]
- 73.Poloczanska ES, Hawkins SJ, Southward AJ, Burrows MT. Modelling the response of populations of competing species to climate change. Ecology. 2008;89:3138–3149. doi: 10.1890/07-1169.1. [DOI] [PubMed] [Google Scholar]
- 74.Hawkins SJ, et al. Complex interactions in a rapidly changing world: responses of rocky shore communities to recent climate change. Clim. Res. 2008;37:123–133. doi: 10.3354/cr00768. [DOI] [Google Scholar]
- 75.Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD. A framework for community interactions under climate change. Trends Ecol. Evol. 2010;25:325–331. doi: 10.1016/j.tree.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 76.Pearce A, Alan P, Feng M. Observations of warming on the Western Australian continental shelf. Mar. Freshwater Res. 2007;58:914–920. doi: 10.1071/MF07082. [DOI] [Google Scholar]
- 77.Watson DL, Harvey ES, Anderson MJ, Kendrick GA. A comparison of temperate reef fish assemblages recorded by three underwater stereo-video techniques. Mar. Biol. 2005;148:415–425. doi: 10.1007/s00227-005-0090-6. [DOI] [Google Scholar]
- 78.Holmes TH, et al. A comparison of visual- and stereo-video based fish community assessment methods in tropical and temperate marine waters of Western Australia. Limnol. Oceanogr. Methods. 2013;11:337–350. doi: 10.4319/lom.2013.11.337. [DOI] [Google Scholar]
- 79.Anderson, M., Gorley, R. N. & Clarke, R. K. Permanova+ for Primer: Guide to Ssoftware and Statistical Methods (PRIMER-E Ltd, 2008).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Documented species of temperate/subtropical and tropical herbivorous fish that consume kelp in different parts of the world.


