Abstract
Objective
We investigated temporal and spatial characteristics of ictal gamma and beta activity on scalp EEG during spasms in patients with West syndrome (WS) to evaluate potential focal cortical onset.
Methods
A total of 1033 spasms from 34 patients with WS of various etiologies were analyzed in video-EEG using time-frequency analysis. Ictal gamma (35–90 Hz) and beta (15–30 Hz) activities were correlated with visual symmetry of spasms, objective EMG (electromyography) analysis, and etiology of WS.
Results
Prior to the ictal motor manifestation, focal ictal gamma activity emerged from one hemisphere (71%, 24/34) or from midline (26%, 9/34), and was rarely simultaneously bilateral (3%, 1/34). Focal ictal beta activity emerged from either one hemisphere (68%, 23/34) or from midline (32%, 11/34). Onsets of focal ictal gamma and beta activity were most commonly observed around the parietal areas. Focal ictal gamma activity propagated faster than ictal beta activity to adjacent electrodes (median: 65 vs. 170 ms, p<0.01), and to contralateral hemisphere (median: 100 vs. 170 ms, p=0.01). Asymmetric peak amplitude of ictal gamma activity in the centroparietal areas (C3-P3 vs. C4-P4) correlated with asymmetric semiology. On the other hand, the majority of visually symmetric spasms showed asymmetry in peak amplitude and interhemispheric onset latency difference in both ictal gamma and beta activity.
Significance
Spasms may be a seizure with focal electrographic onset regardless of visual symmetry. Asymmetric involvement of ictal gamma activity to the centroparietal areas may determine the motor manifestations in WS. Scalp EEG ictal gamma and beta activity may be useful to demonstrate localized seizure onset in infants with WS.
Keywords: West syndrome, high-frequency oscillations (HFOs), gamma activity, beta activity, infantile spasms, seizure semiology
INTRODUCTION
West syndrome (WS) is a rare but devastating epileptic syndrome in infancy with an incidence ranging from 2 to 3.5 per 10,000 live births.1 It is characterized by spasms (frequently in clusters), distinctive interictal and ictal EEG patterns (hypsarrhythmia and electrodecremental response respectively), and often arrest or regression in psychomotor development.1–5 Focal lesions have been associated with infantile spasms and in many cases there has been evidence that focal lesions can generate infantile spasms in humans,6 and in animal models.7, 8 Indeed, the current ‘Operational Classification of Seizure Types by the International League Against Epilepsy’ by Fisher et al. (Epilepsia in press) 9 includes epileptic spasms under both focal and generalized seizures.
The typical ictal EEG finding, the electrodecremental response, consists of widespread slow waves or fast wave bursts regardless of etiology and semiology.10, 11 Observations from humans and animal models of epilepsy suggest that pathological high frequency oscillations (HFOs) with frequencies of 40 Hz or greater may serve as a biomarker of epileptogenicity.12, 13 HFOs can be divided, based on frequency characteristics to gamma (30 – 100 Hz), ripples (100 – 250 Hz), and fast ripples (250 Hz and above).12–14 Also, ictal beta activity (around 20 Hz) during spasms in WS was reported to be associated with seizure recurrence.15
In humans, the detection of HFOs in scalp EEG is challenging.16 HFOs were initially observed with invasive subdural or intracerebral recordings of focal epileptic seizures.17 A prior electrocorticography (ECoG) study demonstrated, during epileptic spasms, recruitment of HFOs in the centroparietal area associated with behavioral spasms involving contraction of the muscles.18 Recent studies showed detectable focal HFOs, especially in the gamma band, around 60 – 90 Hz, on scalp EEG recordings in children with WS.19–21 However, these prior studies only utilized bipolar montages for localization and timing of the HFOs relative to the spasms onset. The use of referential montages is important to evaluate the origin of the activities in each electrode. The aim of this current EEG study was to use quantitative methods to determine whether epileptic spasms show focal activities in beta and gamma bands, identified by onset latencies from referential montages and peak amplitudes from both referential and bipolar montages, and whether this EEG focality is present irrespective of symmetry in spasm semiology.
METHODS
Patients
We retrospectively screened patients with WS from our comprehensive epilepsy database at Montefiore Medical Center consecutively between 2005 and 2014. Inclusion criteria included diagnosis of WS based on video-EEG monitoring and no prior treatment with ACTH and/or other steroids. Prior treatment with antiseizure drugs including vigabatrin (N=1) was not excluded as long as the patient was deemed to be a non-responder at the enrollment during the initial video-EEG monitoring at our institution. The diagnosis of WS was based on the presence of epileptic spasms with EEG evidence of hypsarrhythmia.1–3 Exclusion criteria included either no neuroimaging information or less than 10 spasms with electrodecremental responses captured during video-EEG monitoring, since to perform the time-frequency analysis of EEG data, there must be at least 10 events to statistically evaluate amplitude and latency data.22 The study was approved by the Institutional Review Board at Montefiore Medical Center.
The etiology of WS was classified into known (or presumed genetic/structural-metabolic), or unknown based on a modified version of the recommendations of the International League Against Epilepsy (ILAE) commission on classification and terminology, and National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS) common data elements.23, 24
Video-EEG recordings
Video-EEG was recorded with a digital sampling frequency between 200 Hz and 1024 Hz using a XLTEK digital EEG system (Natus, San Carlos, CA, USA) with the international 10–20 electrode system. EEG/EMG signals were acquired with a low frequency filter (LFF) of 0.1Hz and high frequency filter (HFF) off. The electromyogram (EMG) was simultaneously recorded from two electrodes each placed on the anterior chest wall on either side of the sternum that also allowed the recording of the electrocardiogram (ECG).
Scoring of symmetric vs. asymmetric spasms and voluntary upper limb movements by visual analysis
We identified at least 10 ictal events per patient, each consisting of spasms with electrodecremental response in the conventional video-EEG. EEG and EMG channels were read with filters set at 1 and 70Hz. Spasms were scored as visually symmetric if both sides of the body appeared to be involved with equal intensity at the same time or if symmetric involvement of only axial musculature was noted. The video-EEGs were scored independently by HN and JB and differences in scoring were resolved by additional assessments by ASG and SLM. The scorers were blinded to clinical history and etiology of the patients. Spasms from the same patient were averaged for time-frequency analysis, since the spasms were visually similar (either symmetric or asymmetric).
We also analyzed spontaneous voluntary movements of the upper limbs, unrelated to spasms, for comparison. These movements were collected from interictal periods, visually determined by HN and JB to be clearly different from spasms.
Time-frequency analysis of peri-ictal EEG and EMG data
Visual determination of “zero time point”
The clinical onset of each spasm or spontaneous voluntary movement was identified visually in video recordings and by EMG deflections. Then, the trigger point for time-frequency analyses (set at 0 ms) was set in a temporally expanded trace with LFF 53Hz, HFF off, based on the initial EMG deflection (examples in Figures 1–3), as previously reported.18, 20
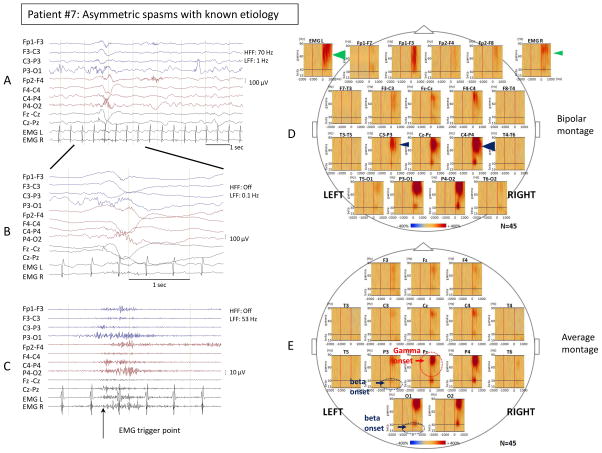
Figure 1. Time-frequency analysis of asymmetric spasms showed focality of ictal gamma and beta activity.
Ictal EEG changes and time-frequency analysis associated with visually asymmetric spasms in patient #7 with spasms of known etiology (bilateral MCA infarcts, L germinal matrix hemorrhage). Sampling frequency: 200 Hz. (A) Bipolar EEG montage. Low frequency filter (LFF): 1 Hz; High frequency filter (HFF): 70 Hz. (B) Temporally expanded trace of panel A. LFF: 0.1 Hz; HFF: off. (C) EEG of panel B filtered with LFF 53 Hz and HFF off. The trigger point for the time-frequency analysis was placed at the EMG onset in the temporally expanded trace. (D) Bipolar montage: Time frequency plots derived from 45 spasms. Please note the maximal augmentation of gamma activity (35–90 Hz) at the centroparietal channels was more prominent on the right side (C4-P4) compared to the left side (C3-P3) (right 639% versus left 251%, blue arrowheads). The maximal augmentation of deflection of the EMG signals was more prominent on the left side compared to the right side (left 267% versus right 187%, green arrowheads). (E) Average montage: Time frequency plots derived from 45 spasms. Statistically determined focal ictal gamma activity (35–90 Hz) onset was at Pz (−260 ms, red circle and arrow), and ictal beta activity (15–30 Hz) onset was at P3 and O1 (−150 ms, blue circle and arrow). In all figures, the blue color indicates attenuation of amplitude, and the red color augmentation of amplitude in the corresponding time-frequency bin relative to the reference period. Please see Supplementary table 2 for detailed values.
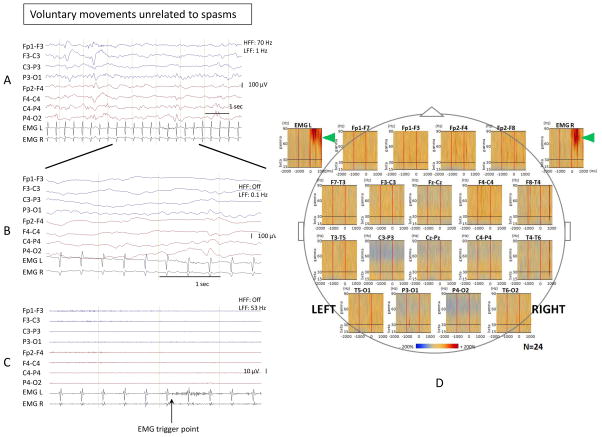
Figure 3. Time-frequency analysis of voluntary movements unrelated to spasms did not show gamma and beta activity.
EEG changes associated with spontaneous voluntary movements unrelated to spasms (brief bilateral upper limb movements) in patient #1 with known etiology (global developmental delay). Sampling frequency: 200 Hz. (A) Bipolar EEG montage. LFF: 1 Hz; HFF: 70 Hz. (B) Temporally expanded trace of panel A. LFF: 0.1 Hz; HFF: off. (C) EEG of panel B filtered with LFF 53 Hz and HFF off. The trigger point for the time-frequency analysis was placed at the EMG onset in the temporally expanded trace. (D) Bipolar montage: Time frequency plots derived from 24 events (spontaneous voluntary movements unrelated to spasms). Augmentation of EMG activity was noted (marked with green arrowheads), but no beta or gamma activity was seen in EEG channels including the centroparietal electrodes.
Automated determination of amplitude augmentations for EEG (beta, gamma) and EMG signals
The EEG and EMG files used for time-frequency analysis were as acquired (LFF 0.1Hz, HFF off). Time-frequency analysis was performed using BESA EEG software (MEGIS Software GmbH, Grafelfing, Germany) and based on the same analysis approaches reported in prior studies.18, 20, 25, 26 We analyzed from −4000 and +2000 ms in 10 ms bins and between 10–90 Hz in 5 Hz bins. The baseline interval was defined as the −4000 ms and −2000 ms epoch. In each patient, the percent change of the root mean square (RMS) of amplitudes (known as the square root of power) with respect to the baseline was averaged over spasms from the same infant and displayed as a function of time and frequency. Here we use “amplitude” instead of “the root mean square (RMS) of amplitudes” for simplification throughout the text.
The ictal frequencies were classified into 2 groups, which were studied separately: beta band (15–30 Hz) and gamma band (35–90 Hz). We investigated the percent increase in amplitude (herein called augmentation of amplitude) of each frequency band in each time bin over its baseline amplitude according to the location of each electrode on a longitudinal bipolar montage and a common average montage (average reference consisted of the mean activity of all cerebral EEG electrodes except Fp1, Fp2, Fpz). For EMG, the augmentation of amplitude was analyzed at a frequency band between 20–90 Hz as previously described.27–27
Spasms in which gamma activity could not be distinguished from artifacts were excluded from the time-frequency analysis (11% were removed). The number of spasms identified and analyzed in each patient is reported in the supplementary data table 1.
Determination of onset latency and peak amplitude
Preliminary results indicated that the onsets of ictal beta and gamma activities were localized at the parasagittal or midline channels. For each of these EEG channels (F3, F4, C3, C4, P3, P4, O1, O2, Fz, Cz, and Pz) and the left and right EMG signals, we extracted the augmentation of amplitude values for the time-period between −4000 ms to +2000 ms. The values were averaged over the beta band (15–30 Hz) and gamma band (35–90 Hz). Onset latency was determined in the average referential montage and was defined as the first time point of the frequency band of interest when [1] the waveform’s augmentation of amplitude exceeded the criterion (defined as “mean+(2.5×SD)” where mean and SD (standard deviation) were computed from the baseline time period −4000 ms to −2000 ms) 28 and [2] the augmentation of amplitude sustained over the criterion for at least 100 ms after this time point (an example shown in supplementary figure 1). The program used to compute onset latencies is included in the supplementary data (onset-latencies_code.R). Onset latencies that corresponded to waveforms without field (i.e., seen in only one channel) were discarded and the next eligible onset was computed. This occurred in only 1 case of computing onset latency for beta and one for gamma frequency bands.
Then, we determined the peak amplitude for both the ictal beta and gamma bands as the maximum augmentation of amplitude value within the time-period −4000 ms to +2000 ms (typically within −2000 ms to +2000 ms period), using average and bipolar montages. Peak amplitude is described as % change from baseline.
Determination of focality of ictal gamma and/or beta onset
We considered that ictal beta and/or gamma activities had focal onset if onsets involved one or two adjacent channels within a hemisphere before spreading. Onsets at 1–2 adjacent electrodes, one of which is midline, may indicate either focal onset or propagation from deep single or bilateral structure(s). For this reason, we analyzed midline onsets separately, although a work up could be considered to look for focal underlying lesions. In this study, we considered the onsets at 2 adjacent channels synchronous when they differed by < 10 ms, because time-frequency analysis was done in 10 ms bins. Analysis of focality of beta and gamma onset was done based on data from the average montage because onset latency cannot be derived from the bipolar montage.
Propagation of ictal gamma and beta
After we determined onset channels using the average montage described above, we calculated the propagation latencies to (a) the contralateral homotypic channel and (b) the adjacent anterior or posterior channel with the faster onset latency in patients with focal hemispheric onset. For patients with midline or bilateral onsets, propagation latency to the contralateral hemisphere was considered as 0 ms. Propagation latencies were defined as the difference in the onset latencies between two channels and computed separately for gamma or beta activity.
Asymmetry index of ictal beta and gamma peak amplitude
Asymmetry of ictal beta and/or gamma peak amplitude was done at both average and bipolar montages, using the asymmetry indices (AI). The AI was defined as: AI = (L−R)/(L+R) 29, where L = averaged augmentation of amplitude in the left side, R = averaged augmentation of amplitude in the right side. The AI was assessed in bipolar channels (F3-C3/F4-C4, C3-P3/C4-P4, and P3-O1/P4-O2), average channels (F3/F4, C3/C4, P3/P4, and O1/O2) and for the augmentation in amplitude of the EMG signals (left EMG/right EMG).
Statistical analyses
Patient characteristics were compared between patients with symmetric versus asymmetric semiology using Mann-Whitney-Wilcoxon test for continuous variables and Pearson’s chi-square test or Fisher’s exact test for categorical variables. Focality data were summarized as median and interquartile range (IQR) of onset latency and peak amplitude, whether the focality could be localized into one hemisphere. The time of propagation from the channel with the earliest onset to the corresponding channel on the contralateral side (homotypic channel) and to the adjacent channels on the ipsilateral side was compared between beta and gamma frequency bands using Wilcoxon signed rank sum test. The symmetry of ictal gamma or beta activity measured by the absolute AI was correlated with the visually determined symmetry of epileptic spasms at each channel. The association between peak amplitude of beta and gamma activities and age was assessed separately for each frequency band. All statistical analyses were performed using the statistical software R (version 3.2.0; http://cran.r-project.org).
RESULTS
Patient characteristics
From 53 patients who were screened, 19 patients were excluded due to less than 10 analyzable events on available video-EEG recordings. The study cohort consisted of 34 patients (median age 6.9 months, range 3.0 – 23.9 months; 10 females/24 males; 19 known/15 unknown etiology: Table 1). The patients who were excluded due to insufficient video-EEG recording did not differ from the study subjects in terms of patients’ characteristics, (median age 7.8 months, ranged 2.9 – 24 months [p=0.44, T-test]; 10 females/9 males [p=0.14, Fisher’s exact test]; 11 known/8 unknown etiology [p=0.92, Fisher’s exact test]).
Table 1.
Summary of data in asymmetric and symmetric spasms a
| Whole sample (n=34) | Asymmetric semiology (n=10) | Symmetric semiology (n=24) | Asymmetric vs. Symmetric P-value b | |
|---|---|---|---|---|
| Age, months | 6.9 (5.7, 9.6) | 6.5 (4.6, 13.0) | 7.0 (5.8, 8.7) | 0.86 |
| Female (%) | 10 (29%) | 3 (30%) | 7 (29%) | 1.00 |
| Etiologies – Known (%) | 19 (56%) | 9 (90%) | 10 (42%) | 0.02 |
| Onset latency of gamma, ms | −450 (−543, −265) | −260 (−480, −213) | −455 (−568, −358) | 0.11 |
| Onset latency of beta, ms | −565 (−663, −338) | −500 (−925, −248) | −565 (−648, −383) | 0.88 |
| Peak amplitude of gamma (%) | 374 (250, 523) | 429 (268, 523) | 364 (255, 505) | 0.75 |
| Peak amplitude of beta (%) | 300 (220, 389) | 263 (202, 398) | 300 (246, 388) | 0.59 |
| Onset of ictal gamma and beta activity lateralized to the same hemisphere (%) c | 10/16 (63%) | 2/4 (50%) | 8/12 (67%) | 1.00 |
| Peak amplitude of ictal gamma and beta activity lateralized to the same hemisphere (%) | 23 (68%) | 7 (70%) | 16 (67%) | 1.00 |
Continuous variables are summarized as median (Inter-quartile range, IQR) while categorical variables are summarized as frequency counts (proportions).
Continuous variables were compared using Mann-Whitney-Wilcoxon test and categorical variables were compared using Pearson’s chi-square test or Fisher’s exact test.
16 out of 34 patients showed focal onset (exclusively hemispheric) in both gamma and beta activity.
Visual analysis of spasms
The median number of spasms analyzed per patient was 24 (1033 in total, range: 17 – 72 spasms/patient) (supplementary table 1). In the first round of review by HN and JB, there was good initial agreement in 30 out of 35 spasm types (88.6% agreement, Cohen’s kappa=0.68) and after discussion, consensus was reached in all. The 35 spasm types for 34 patients were due to one patient being scored having two different types of spasms by HN and JB. Additional assessments by ASG and SLM suggested that these two different types of spasms had the same semiology. Our final consensus was that each individual patient had only one semiology of the spasms. Asymmetric spasms were seen in 9/19 (47%) of patients with known etiology but in only 1/15 (7%) infants with spasms of unknown etiology (p=0.02, Fisher’s exact test).
Time-frequency analysis of peri-ictal EEG and EMG data
Ictal gamma and beta EEG activities were present and temporally associated with spasms in all cases and were best seen in the temporally expanded EEG traces without filters (examples in Figure 1B and 2B). These activities subsided without evolving into other forms of ictal discharges. In each spasm, ictal gamma and beta activities preceded the EMG signals associated with motor manifestations and their waveforms and frequency characteristics were different from muscle activity in the EMG (Figure 1 and 2). During voluntary movements unrelated to spasms, there was no gamma or beta activity observed in the temporally expanded EEG traces or on time-frequency analysis (Figure 3).
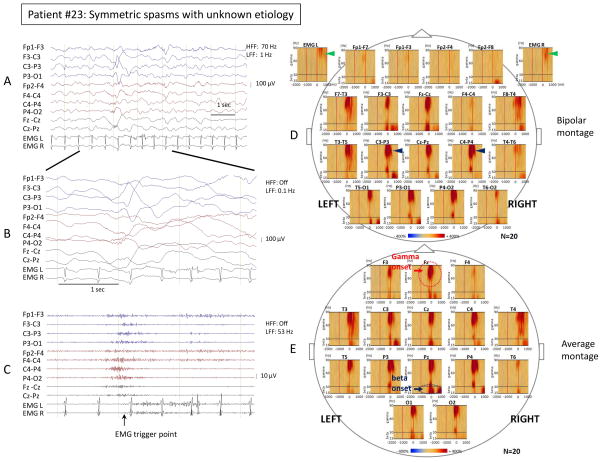
Figure 2. Time-frequency analysis of symmetric spasms showed focality of ictal gamma and beta activity.
Ictal EEG changes and time-frequency analysis associated with visually symmetric spasms in patient #23 with unknown etiology. Sampling frequency: 200 Hz. (A) Bipolar EEG montage. LFF: 1 Hz; HFF: 70 Hz. (B) Temporally expanded trace of panel A. LFF: 0.1 Hz; HFF: off. (C) EEG of panel B filtered with LFF 53 Hz and HFF off. The trigger point for the time-frequency analysis was placed at the EMG onset. (D) Bipolar montage: Time frequency plots derived from 20 spasms. Please note the maximal augmentation of gamma activity (35–90 Hz) at the centroparietal electrodes was almost equal between the left side (C3-P3) and the right side (C4-P4) (left 651% versus right 717%, blue arrowheads). The maximal augmentation of deflection of the EMG signals was almost equal between the left side and the right side (left 192% versus right 178%, green arrowheads). (E) Average montage: Time frequency plots derived from 20 spasms. Statistically determined focal ictal gamma activity onset was at Fz (−590 ms, red circle and arrow), and ictal beta activity onset was also at Pz (−590 ms, blue circle and arrow).
Ictal gamma and beta activity showed focality, most commonly in the parietal area in both asymmetric and symmetric spasms
Both ictal gamma and beta activity occurred prior to the EMG onset [median (IQR): −450ms (−543, −265) for gamma vs. −565ms (−663, −338) for beta]. Regarding ictal gamma activity, focal onset (exclusively hemispheric onset) was seen in 24 out of 34 patients (71%), midline onset was seen in 9 patients (26%), and bilateral onset was seen in 1 patient (3%). Regarding ictal beta activity, focal onset was seen in 23 out of 34 patients (68%), midline onset was seen in 11 patients (32%), and bilateral onset was not seen in any of the patients (0%). The focal onset of ictal gamma activity was most commonly observed in parietal channel (10/24, 42%) and less commonly observed in frontal (3/24, 13%), central (5/24, 21%), and occipital (6/24, 25%) channels. The focal onset of ictal beta activity was most commonly observed in parietal (7/23, 30%), then occipital (6/23, 26%), and less commonly observed in frontal (3/23, 13%) and central (4/23, 17%) channels. Of note, two patients showed simultaneous onset at parietal and occipital channels (2/23, 9%), and one patient showed simultaneous onset at central and occipital channels (1/23, 4%) [detailed focality data for each patient are shown in Supplementary Table 2]. Localization of gamma or beta activities did not correlate with the presence or absence of MRI abnormalities (Supplementary table 3).
Regarding the patients showing focal onset, the focal ictal gamma activity propagated from the onset channel to adjacent channels faster than ictal beta activity [median (IQR): 65ms (37.5, 102.5) vs. 170ms (85, 410), p<0.01]. Also, the focal ictal gamma activity propagated from the onset channel to the contralateral hemisphere faster than ictal beta activity [median (IQR): 100ms (60, 170) vs. 170 ms (115, 265), p=0.01]. Detailed data on onset latency difference between hemispheres in each patient are shown in Figure 4.
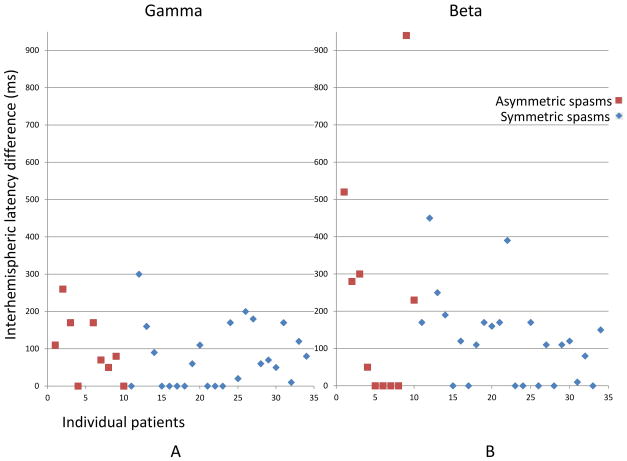
Figure 4. Interhemispheric onset latency difference showed evidence of focality in most spasms, regardless of visual symmetry.
Propagation latency to contralateral hemisphere (=onset latency of the homotypic contralateral electrode - onset latency of the onset electrode) was plotted in each patient. Onset latency was plotted as 0 ms if onset was bilateral or midline. In X axis, 1–10 represent patients with asymmetric spasms (brown squares), and 11–34 represent patients with symmetric spasms (blue rhombi). Please note these patient numbers do not correspond to patient numbers in Supplementary table 1 or prior figures. In Y axis, interhemispheric onset latency difference (ms) is shown. Please also note that the gamma onset latency of the homotypic contralateral electrode in patient #9 (corresponding to X=5 in this figure) could not be determined (the peak did not reach more than mean +2SD), so there were only 9 brown squares shown in gamma, but 10 were shown in beta.
Asymmetric ictal gamma activity in the centroparietal areas correlates to asymmetric semiology
Absolute asymmetry indexes (AIs) of peak amplitude of ictal gamma activity in the centroparietal channels (C3-P3 vs. C4-P4) in the bipolar montage correlated with visual asymmetry of the spasms (p=0.001) (Table 2). There was similar trend in relationship between absolute AIs of peak amplitude of ictal gamma activity in the central channels (C3 vs. C4) in the average montage and visual asymmetry of the spasms, but it did not reach statistical significance (p=0.07). The absolute AIs of ictal beta activity did not significantly correlate with visual symmetry in any of the channels.
Table 2.
Ictal gamma and beta activity in relation to visual symmetry of spasms
| Gamma amplitude difference | |||
|---|---|---|---|
| Electrode | Semiology | Median Abs AI [Q1, Q3] | p value |
| F3 vs F4 | asym | 0.18 [0.11, 0.25] | 0.89 |
| sym | 0.16 [0.06, 0.32] | ||
| C3 vs C4 | asym | 0.19 [0.08, 0.28] | 0.07 |
| sym | 0.07 [0.03, 0.14] | ||
| P3 vs P4 | asym | 0.08 [0.02, 0.23] | 0.90 |
| sym | 0.08 [0.06, 0.13] | ||
| O1 vs O2 | asym | 0.11 [0.02, 0.23] | 0.95 |
| sym | 0.10 [0.06, 0.17] | ||
| F3-C3 vs F4-C4 | asym | 0.27 [0.15, 0.37] | 0.30 |
| sym | 0.15 [0.10, 0.31] | ||
| C3-P3 vs C4-P4 | asym | 0.27 [0.15, 0.33] | 0.001 |
| sym | 0.06 [0.03, 0.14] | ||
| P3-O1 vs P4-O2 | asym | 0.10 [0.06, 0.20] | 0.90 |
| sym | 0.11 [0.04, 0.16] | ||
| Beta amplitude difference | |||
|---|---|---|---|
| Electrode | Semiology | Median Abs AI [Q1, Q3] | p value |
| F3 vs F4 | asym | 0.16 [0.08, 0.32] | 0.32 |
| sym | 0.12 [0.06, 0.16] | ||
| C3 vs C4 | asym | 0.09 [0.03, 0.17] | 0.11 |
| sym | 0.18 [0.08, 0.23] | ||
| P3 vs P4 | asym | 0.15 [0.10, 0.20] | 0.25 |
| sym | 0.12 [0.04, 0.22] | ||
| O1 vs O2 | asym | 0.07 [0.04, 0.19] | 0.90 |
| sym | 0.10 [0.04, 0.17] | ||
| F3-C3 vs F4-C4 | asym | 0.26 [0.13, 0.41] | 0.06 |
| sym | 0.10 [0.05, 0.26] | ||
| C3-P3 vs C4-P4 | asym | 0.07 [0.02, 0.21] | 0.47 |
| sym | 0.11 [0.07, 0.26] | ||
| P3-O1 vs P4-O2 | asym | 0.11 [0.09, 0.19] | 0.78 |
| sym | 0.14 [0.07, 0.21] | ||
Abs AI: Absolute asymmetry index.
Peak amplitude of ictal gamma and beta activity is more prominent in younger children
Polynomial (second order) regression analysis demonstrated that the maximal augmentation of gamma activity (averaged at parietal channels) was more prominent in younger children (R=−0.42, p=0.03). Although the maximal augmentation of beta activity showed similar pattern, it did not reach statistical significance (R=−0.26, p=0.28) (Supplementary figure 2).
DISCUSSION
This study demonstrates that ictal gamma and beta activity can be visually identified and quantified by time-frequency analyses in scalp EEG recordings. Our data suggest that most epileptic spasms may demonstrate focal onset ictal gamma and beta activity regardless of the visual symmetry of their semiology. Spontaneous voluntary movements unrelated to spasms (brief upper limb movements) were not associated with gamma or beta activity.
Focal seizures are defined as originating within networks limited to one hemisphere and either discretely localized or more widely distributed 23. In this study, we defined that ictal beta and/or gamma activities had focal onset if onsets involved one or two adjacent electrodes within a hemisphere and considered separately the patients who showed onset at a midline electrode. Based on this definition, most patients showed focality in a unilateral hemisphere. Of those with midline onset, we recognize that they may either represent focal-onset seizures or propagation from deep structure(s) whether unilateral, bilateral or midline. One patient (patient #10) had bilateral gamma onset and focal beta onset and had symmetric spasms and evidence of partial agenesis of corpus callosum. Future studies will reveal whether focality may or may not underlie the epilepsy in this patient. The ultimate gold standard of a focal epileptic zone is seizure freedom after surgery. While none of our patients had surgery yet, our data point to a focality and further substantiate the need for more aggressive approaches to identify a focus even when the symptoms are bilateral as in epileptic spasms. Indeed, the current ‘Operational Classification of Seizure Types by the International League Against Epilepsy’ by Fisher et al (Epilepsia in press) 9 includes spasms under both focal and generalized seizures.
However, the definition of the focality is arbitrary, when we consider that lateralized focal onset demonstrates a difference in onset latency of ictal gamma and beta activity equal or larger than 10 ms between hemispheres. If the difference in onset latency between hemispheres was defined to be equal or larger than 100 ms, focality of ictal gamma activity would have been seen in 12/34 (35%) and that of beta activity in 20/34 (59%).
Generalized seizures are defined as occurring in and rapidly engaging bilaterally distributing networks 23. While ictal gamma and beta activity eventually involved both hemispheres in all our cases, this study demonstrated that the focal ictal gamma and beta activity propagated from the onset electrode to the contralateral hemisphere with a significant latency delay (gamma: median 100 ms, beta: median 170 ms). Even in visually symmetric spasms, the absolute AI of gamma activity was 0.06 at centroparietal channels, which means 11% difference of amplitude between hemispheres. This suggests there is asymmetry in EEG signal even in visually symmetric spasms.
Although previous studies reported the presence of scalp EEG beta activity 15 and gamma activity 19, 30 during spasms, there are no studies with quantified data correlating specific frequency bands in specific EEG electrodes to clinical manifestations using both referential and bipolar montages. Indeed, the use of both montages offers complementary information.
The present study demonstrated that ictal augmentation of gamma and beta activity consistently preceded clinical onset. Previous studies have reported that unilateral ictal EEG changes were associated with contralateral motor manifestations in WS with known etiologies,31, 32 which suggested underlying focal pathology in WS. Our data support these findings and show that asymmetric ictal gamma activity in the centroparietal area is associated with observable asymmetry of the spasms. This relationship was not observed in ictal beta activity or in sites other than the centroparietal areas. Ictal gamma rather than beta activity might be a stronger determinant in semiology of behavioral epileptic spasms.
There are still some technical limitations that need to be considered. We evaluated symmetry of spasms using deflection of the EMG signals at the anterior chest electrodes; however, this is probably not sufficient to pick up subtle asymmetry of the distal upper limb movements, the lower limb movements, or neck movements. Also, the initial deflection of the EMG signals was considered as the initial motor manifestation; this method might not have picked up subtle motor onset or initial ictal motor phenomena at other muscle groups. Polygraphic monitoring with electromyogram, eye channels and accelerometer channels could be used to describe seizure semiology more accurately.
Time-frequency analysis was performed in each spasm and analysis was done in the averaged events as a group. This approach appears to be clinically relevant, since semiology throughout a cluster usually shows consistently similar motor manifestations 32. However, there is a limitation in addressing consistency in each spasm. Spasms were analyzed as a whole (time-frequency analysis was done in each spasm but analysis was done in the averaged events) to reduce the possibility that random elevations of noise may contribute to “false starts”. The definition of ictal onset (gamma or beta) also included a “duration for more than 100 ms” criterion, to avoid detection of random peaks.
Nine out of ten patients showing clinically asymmetric spasms had WS with known etiology, which supports the previous findings that asymmetric spasms are seen more in symptomatic WS using an earlier classification scheme.11
The data also demonstrated that the maximal augmentation of ictal gamma activity was negatively correlated with age. It may reflect the fact that younger children have thin skull thickness which allows more sampling of higher frequency activity. Our polynomial regression model suggests this curve may plateau through the older ages (although the number of older children was limited in the cohort). The relatively low R values can be explained by the fewer cases of WS in the older age groups. Future studies including more patients may help refine the correlation of ictal gamma and beta augmentation with age. Since WS is an age-specific disorder, specific augmentation of ictal gamma activity in younger children may serve as a clue to elucidate the pathophysiology of generation of spasms. High frequency EEG activity, such as gamma activity has been found most often in the cortical regions where seizures originate and rarely in regions to which they propagate. 12 Functional imaging studies (PET and SPECT) suggested involvement of the basal ganglia and brainstem during spasms 33, 34. This can be a secondary phenomenon associated with the spread of the cortical foci to subcortical structure 35, although it is also likely that basal ganglia and/or brainstem lesions may be generating spasms, as suggested by the occasional reports of hydranencephaly associated with spasms 36. It is therefore possible, that in certain cases, the focally identified gamma activity may represent propagation of ictal activity from subcortical structures, but this cannot be tested with surface EEG. However, studies from animal models indicate that focal lesions can induce spasms7. Focality of surface EEG in epileptic spasms with non-lesional MRI should be considered. Previous studies suggested focality in drug resistant patients with WS who are considered for possible epilepsy surgery,37 however this study suggests that evidence of focality may be already present during first diagnosis in the majority of infants with WS.
HFOs are considered important in the generation of seizures.12, 38, 39 Previous ECoG (electrocorticography) studies demonstrated that removing cortical regions showing more HFOs were associated with good seizure outcome.18, 40–42 When present, asymmetry of ictal gamma or beta activity may provide additional evidence of electroclinical asymmetry that may be worth investigating further for the localization of a possible underlying focus.
The current study investigated the frequency band up to gamma range (up to 90 Hz). It included patients with higher sampling frequency up to 1024 Hz as well as patients sampled at a frequency of 200 Hz. Based on preliminary analysis, the mean onset frequency of ictal activity was 68.53 Hz with 200 Hz sampling frequency (n=17), and that with 1024 Hz sampling frequency was 68.57 Hz (n=7) (p=0.63, Mann Whitney U test). Similarly, there were no difference in the mean maximal augmentation of gamma activity (35–90 Hz) at the Rolandic electrodes (C3-P3, C4-P4) between subjects with 200 Hz sampling frequency of 470.3% and subjects with 1024 Hz sampling frequency of 423.5% (p=0.31, Mann Whitney U test). Ripple band (100 Hz and above) was present in such patients with higher sampling frequency band, but such activity was not prominent compared to gamma band. Based on the above pilot data, analysis was done on frequencies up to 90 Hz, so that patients with sampling frequency of 200 Hz could be included in the analysis as a single group. Our pilot findings were consistent with a previous study in which ictal gamma activity during spasms was around 65 Hz, ranging from 51–98 Hz, using sampling frequency of 500 Hz.19 The recent study using sampling frequency with 500 Hz reported interictal and ictal activity up to 150 Hz (ripple band) in patients with WS.30 However, higher number of study patients recorded with higher sampling frequencies would be needed to study ripples.
In future studies, the role of faster (ripple or fast ripple band) or lower frequency bands will be assessed to determine their contribution to the identification of a focal lesion. Such identification will help to better define the pathophysiology of this devastating disease, and may lead to improved treatments, including surgical interventions.
Supplementary Material
Ictal EEG changes and determination of onset latency of ictal gamma and beta activity in patient #23 with spasms of unknown etiology. Sampling frequency: 200 Hz. (A) Common average montage. Low frequency filter (LFF): 1 Hz; High frequency filter (HFF): 70 Hz. (B) EEG of panel A filtered with LFF 53 Hz and HFF off. The trigger point for the time-frequency analysis was placed at the EMG onset in the temporally expanded trace. Based on the trigger point, the baseline time-period −4000 ms to −2000 ms is determined during analysis period −4000 ms to +2000 ms. (C) Determination of onset latency of ictal gamma activity. In each EEG channel (F3, F4, C3, C4, P3, P4, O1, O2, Fz, Cz, and Pz), onset latency is determined at the first time point of the gamma band when [1] the waveform’s amplitude exceeded the criterion (defined as “mean+(2.5×SD)” where mean and SD were computed from the baseline and [2] the amplitude sustained over the criterion for at least 100 ms after this time point. (D) Determination of onset latency of ictal beta activity is performed as described above.
(A) Polynomial (second order) regression analysis demonstrated the averaged maximal augmentation of gamma activity at the parietal channels negatively correlated with age (R=−0.42, p=0.03) regardless of visual symmetry of spasms. (B) Polynomial (second order) regression analysis demonstrated the averaged maximal augmentation of beta activity at the parietal channels showed similar pattern towards negative correlation with age, but failed to show statistical significance (Pearson R=−0.26, p=0.28). Patients with asymmetric spasms are shown in brown squares, and those with symmetric spasms are shown in blue rhombi.
KEY BULLET POINTS.
Focal or midline onsets of ictal gamma and beta activities were observed prior to the ictal motor manifestations regardless of visual symmetry of spasms.
Onsets of focal ictal gamma and beta activity were most commonly observed around the parietal areas.
Focal ictal gamma activity propagated faster than ictal beta activity to adjacent electrodes, and to contralateral hemisphere.
Asymmetric peak amplitude of ictal gamma activity in the centroparietal areas (C3-P3 vs. C4-P4) correlated with asymmetric semiology.
Even in visually symmetric spasms, there was asymmetry in peak amplitude of ictal gamma and beta activity.
Acknowledgments
We are grateful to Fred Lado and staff members of The Comprehensive Epilepsy Center at Montefiore Medical Center for the collaboration and assistance in performing the studies described herein.
Solomon L. Moshé is the Charles Frost Chair in Neurosurgery and Neurology and funded by grants from NIH NS43209, NS20253, NS45911, NS-78333, 1U54NS100064-01, CURE Infantile Spasms Initiative, US Department of Defense (W81XWH-13-1-0180), the Heffer Family and the Segal Family Foundations and the Abbe Goldstein/Joshua Lurie and Laurie Marsh/Dan Levitz families. He is serving as Associate Editor of Neurobiology of Disease, and is on the editorial board of Epileptic Disorders, Brain and Development, Pediatric Neurology and Physiological Research. He receives from Elsevier an annual compensation for his work as Associate Editor in Neurobiology of Disease and royalties from 2 books he co-edited. He received a consultant fee from Eisai and UCB.
Aristea S. Galanopoulou receives research funding from NINDS NS091170, 1U54NS100064-01, US Department of Defense (W81XWH-13-1-0180), CURE Infantile Spasms Initiative, the Heffer Family and the Segal Family Foundations and the Abbe Goldstein/Joshua Lurie and Laurie Marsh/Dan Levitz families. She has received honoraria from the Department of Defense (grant reviews), John Libbey Eurotext and Elsevier (publications).
Wenzhu B. Mowrey receives research funding from US Department of Defense (W81XWH-13-1-0180), NINDS 1U54NS100064-01, and CURE Infantile Spasms Initiative.
Shlomo Shinnar MD PhD is funded by NIH grants NS 2R37-NS043209, 2U01-NS045911, U10NS077308, and 1U-1NS08803. He serves on the Editorial Board of Pediatric Neurology and serves on DSMBs for UCB Pharma. He has received personal compensation for serving on Scientific Advisory Boards for, UCB Pharma and Upsher Smith and for consulting for Neurelis, Questcor, Upsher-Smith and Xeris. He has received royalties from Elsevier for co-editing the book Febrile Seizures.
References
- 1.Pellock JM, Hrachovy R, Shinnar S, et al. Infantile spasms: a U.S. consensus report. Epilepsia. 2010;51:2175–2189. doi: 10.1111/j.1528-1167.2010.02657.x. [DOI] [PubMed] [Google Scholar]
- 2.Lux AL, Osborne JP. A proposal for case definitions and outcome measures in studies of infantile spasms and West syndrome: consensus statement of the West Delphi group. Epilepsia. 2004;45:1416–1428. doi: 10.1111/j.0013-9580.2004.02404.x. [DOI] [PubMed] [Google Scholar]
- 3.Hrachovy RA, Frost JD., Jr Infantile spasms. Handb Clin Neurol. 2013;111:611–618. doi: 10.1016/B978-0-444-52891-9.00063-4. [DOI] [PubMed] [Google Scholar]
- 4.Karvelas G, Lortie A, Scantlebury MH, et al. A retrospective study on aetiology based outcome of infantile spasms. Seizure. 2009;18:197–201. doi: 10.1016/j.seizure.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Vendrame M, Guilhoto LM, Loddenkemper T, et al. Outcomes of epileptic spasms in patients aged less than 3 years: single-center United States experience. Pediatr Neurol. 2012;46:276–280. doi: 10.1016/j.pediatrneurol.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Chugani HT, Shields WD, Shewmon DA, et al. Infantile spasms: I. PET identifies focal cortical dysgenesis in cryptogenic cases for surgical treatment. Ann Neurol. 1990;27:406–413. doi: 10.1002/ana.410270408. [DOI] [PubMed] [Google Scholar]
- 7.Scantlebury MH, Galanopoulou AS, Chudomelova L, et al. A model of symptomatic infantile spasms syndrome. Neurobiol Dis. 2010;37:604–612. doi: 10.1016/j.nbd.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CL, Frost JD, Jr, Swann JW, et al. A new animal model of infantile spasms with unprovoked persistent seizures. Epilepsia. 2008;49:298–307. doi: 10.1111/j.1528-1167.2007.01377.x. [DOI] [PubMed] [Google Scholar]
- 9.Fisher R, Cross J, French J, et al. Operational Classification of Seizure Types by the International League Against Epilepsy. Epilepsia. 2017 doi: 10.1111/epi.13670. In press. [DOI] [PubMed] [Google Scholar]
- 10.Kellaway P, Hrachovy RA, Frost JD, Jr, et al. Precise characterization and quantification of infantile spasms. Ann Neurol. 1979;6:214–218. doi: 10.1002/ana.410060306. [DOI] [PubMed] [Google Scholar]
- 11.Fusco L, Vigevano F. Ictal clinical electroencephalographic findings of spasms in West syndrome. Epilepsia. 1993;34:671–678. doi: 10.1111/j.1528-1157.1993.tb00445.x. [DOI] [PubMed] [Google Scholar]
- 12.Gotman J. High frequency oscillations: the new EEG frontier? Epilepsia. 2010;51(Suppl 1):63–65. doi: 10.1111/j.1528-1167.2009.02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zijlmans M, Jiruska P, Zelmann R, et al. High-frequency oscillations as a new biomarker in epilepsy. Ann Neurol. 2012;71:169–178. doi: 10.1002/ana.22548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jirsch JD, Urrestarazu E, LeVan P, et al. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593–1608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- 15.Panzica F, Binelli S, Canafoglia L, et al. ICTAL EEG fast activity in West syndrome: from onset to outcome. Epilepsia. 2007;48:2101–2110. doi: 10.1111/j.1528-1167.2007.01264.x. [DOI] [PubMed] [Google Scholar]
- 16.Michel CM, Murray MM. Discussing gamma. Brain Topogr. 2009;22:1–2. doi: 10.1007/s10548-009-0082-9. [DOI] [PubMed] [Google Scholar]
- 17.Fisher RS, Webber WR, Lesser RP, et al. High-frequency EEG activity at the start of seizures. J Clin Neurophysiol. 1992;9:441–448. doi: 10.1097/00004691-199207010-00012. [DOI] [PubMed] [Google Scholar]
- 18.Nariai H, Nagasawa T, Juhasz C, et al. Statistical mapping of ictal high-frequency oscillations in epileptic spasms. Epilepsia. 2011;52:63–74. doi: 10.1111/j.1528-1167.2010.02786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi K, Oka M, Akiyama T, et al. Very fast rhythmic activity on scalp EEG associated with epileptic spasms. Epilepsia. 2004;45:488–496. doi: 10.1111/j.0013-9580.2004.45703.x. [DOI] [PubMed] [Google Scholar]
- 20.Iwatani Y, Kagitani-Shimono K, Tominaga K, et al. Ictal high-frequency oscillations on scalp EEG recordings in symptomatic West syndrome. Epilepsy Res. 2012;102:60–70. doi: 10.1016/j.eplepsyres.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi K, Akiyama T, Oka M, et al. A storm of fast (40–150Hz) oscillations during hypsarrhythmia in West syndrome. Ann Neurol. 2015;77:58–67. doi: 10.1002/ana.24299. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi K, Yoshinaga H, Toda Y, et al. High-frequency oscillations in idiopathic partial epilepsy of childhood. Epilepsia. 2011;52:1812–1819. doi: 10.1111/j.1528-1167.2011.03169.x. [DOI] [PubMed] [Google Scholar]
- 23.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 24.Loring DW, Lowenstein DH, Barbaro NM, et al. Common data elements in epilepsy research: development and implementation of the NINDS epilepsy CDE project. Epilepsia. 2011;52:1186–1191. doi: 10.1111/j.1528-1167.2011.03018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoechstetter K, Bornfleth H, Weckesser D, et al. BESA source coherence: a new method to study cortical oscillatory coupling. Brain Topogr. 2004;16:233–238. doi: 10.1023/b:brat.0000032857.55223.5d. [DOI] [PubMed] [Google Scholar]
- 26.Papp N, Ktonas P. Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed Sci Instrum. 1977;13:135–145. [PubMed] [Google Scholar]
- 27.Chowdhury RH, Reaz MB, Ali MA, et al. Surface electromyography signal processing and classification techniques. Sensors (Basel) 2013;13:12431–12466. doi: 10.3390/s130912431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osman A, Bashore TR, Coles MG, et al. On the transmission of partial information: inferences from movement-related brain potentials. J Exp Psychol Hum Percept Perform. 1992;18:217–232. doi: 10.1037//0096-1523.18.1.217. [DOI] [PubMed] [Google Scholar]
- 29.Lyamin OI, Pavlova IF, Kosenko PO, et al. Regional differences in cortical electroencephalogram (EEG) slow wave activity and interhemispheric EEG asymmetry in the fur seal. J Sleep Res. 2012;21:603–611. doi: 10.1111/j.1365-2869.2012.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi K, Akiyama T, Oka M, et al. A storm of fast (40–150Hz) oscillations during hypsarrhythmia in West syndrome. Ann Neurol. 2014 doi: 10.1002/ana.24299. [DOI] [PubMed] [Google Scholar]
- 31.Gaily EK, Shewmon DA, Chugani HT, et al. Asymmetric and asynchronous infantile spasms. Epilepsia. 1995;36:873–882. doi: 10.1111/j.1528-1157.1995.tb01630.x. [DOI] [PubMed] [Google Scholar]
- 32.Bisulli F, Volpi L, Meletti S, et al. Ictal pattern of EEG and muscular activation in symptomatic infantile spasms: a videopolygraphic and computer analysis. Epilepsia. 2002;43:1559–1563. doi: 10.1046/j.1528-1157.2002.15302.x. [DOI] [PubMed] [Google Scholar]
- 33.Chugani HT, Shewmon DA, Sankar R, et al. Infantile spasms: II. Lenticular nuclei and brain stem activation on positron emission tomography. Ann Neurol. 1992;31:212–219. doi: 10.1002/ana.410310212. [DOI] [PubMed] [Google Scholar]
- 34.Munakata M, Haginoya K, Ishitobi M, et al. Dynamic cortical activity during spasms in three patients with West syndrome: a multichannel near-infrared spectroscopic topography study. Epilepsia. 2004;45:1248–1257. doi: 10.1111/j.0013-9580.2004.t01-1-04004.x. [DOI] [PubMed] [Google Scholar]
- 35.Lado FA, Rubboli G, Capovilla G, et al. Pathophysiology of epileptic encephalopathies. Epilepsia. 2013;54(Suppl 8):6–13. doi: 10.1111/epi.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neville BG. The origin of infantile spasms: evidence from a case of hydranencephaly. Dev Med Child Neurol. 1972;14:644–647. doi: 10.1111/j.1469-8749.1972.tb02647.x. [DOI] [PubMed] [Google Scholar]
- 37.Chugani HT, Shewmon DA, Shields WD, et al. Surgery for intractable infantile spasms: neuroimaging perspectives. Epilepsia. 1993;34:764–771. doi: 10.1111/j.1528-1157.1993.tb00459.x. [DOI] [PubMed] [Google Scholar]
- 38.Wendling F, Bartolomei F, Bellanger JJ, et al. Epileptic fast intracerebral EEG activity: evidence for spatial decorrelation at seizure onset. Brain. 2003;126:1449–1459. doi: 10.1093/brain/awg144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zijlmans M, Jacobs J, Zelmann R, et al. High-frequency oscillations mirror disease activity in patients with epilepsy. Neurology. 2009;72:979–986. doi: 10.1212/01.wnl.0000344402.20334.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akiyama T, McCoy B, Go CY, et al. Focal resection of fast ripples on extraoperative intracranial EEG improves seizure outcome in pediatric epilepsy. Epilepsia. 2011;52:1802–1811. doi: 10.1111/j.1528-1167.2011.03199.x. [DOI] [PubMed] [Google Scholar]
- 41.Jacobs J, Zijlmans M, Zelmann R, et al. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol. 2010;67:209–220. doi: 10.1002/ana.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ochi A, Otsubo H, Donner EJ, et al. Dynamic changes of ictal high-frequency oscillations in neocortical epilepsy: using multiple band frequency analysis. Epilepsia. 2007;48:286–296. doi: 10.1111/j.1528-1167.2007.00923.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ictal EEG changes and determination of onset latency of ictal gamma and beta activity in patient #23 with spasms of unknown etiology. Sampling frequency: 200 Hz. (A) Common average montage. Low frequency filter (LFF): 1 Hz; High frequency filter (HFF): 70 Hz. (B) EEG of panel A filtered with LFF 53 Hz and HFF off. The trigger point for the time-frequency analysis was placed at the EMG onset in the temporally expanded trace. Based on the trigger point, the baseline time-period −4000 ms to −2000 ms is determined during analysis period −4000 ms to +2000 ms. (C) Determination of onset latency of ictal gamma activity. In each EEG channel (F3, F4, C3, C4, P3, P4, O1, O2, Fz, Cz, and Pz), onset latency is determined at the first time point of the gamma band when [1] the waveform’s amplitude exceeded the criterion (defined as “mean+(2.5×SD)” where mean and SD were computed from the baseline and [2] the amplitude sustained over the criterion for at least 100 ms after this time point. (D) Determination of onset latency of ictal beta activity is performed as described above.
(A) Polynomial (second order) regression analysis demonstrated the averaged maximal augmentation of gamma activity at the parietal channels negatively correlated with age (R=−0.42, p=0.03) regardless of visual symmetry of spasms. (B) Polynomial (second order) regression analysis demonstrated the averaged maximal augmentation of beta activity at the parietal channels showed similar pattern towards negative correlation with age, but failed to show statistical significance (Pearson R=−0.26, p=0.28). Patients with asymmetric spasms are shown in brown squares, and those with symmetric spasms are shown in blue rhombi.