FIGURE 3.
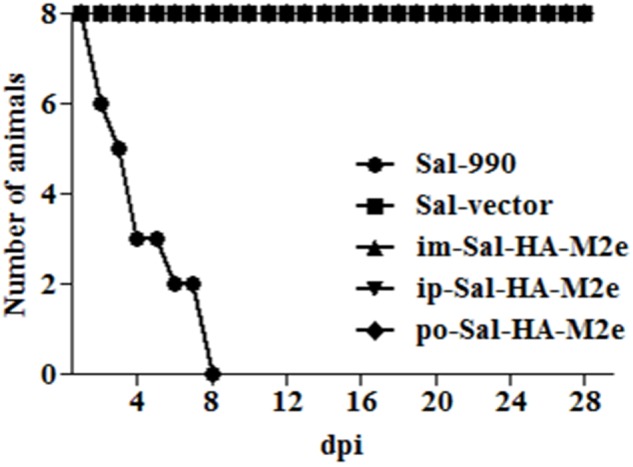
In vivo safety evaluation of the vaccine. Mice were either inoculated intramuscularly with the wild type strain JOL990 (Sal-990, n = 8), PBS only, JOL1837 carrying empty vector (Sal-vector) and constructs JOL1917+1913 (im-Sal-HA-M2e) or intraperitoneally with constructs JOL1913+1917 (ip-Sal-HA-M2e) or orally with constructs JOL1913+1917 (po-Sal-HA-M2e). The animals were monitored for 28 days and the mortality of the animals was recorded. Non-lethal endpoints were used during the experiments and mice were euthanized to limit suffering, when they exhibited weight loss of more than 30%, lethargy, ruffled hair coat, or hunched posture.
