Abstract
Selected functional properties of four types of gluten-free muffins made of unfermented and fermented (by Lactobacillus plantarum) buckwheat flour in comparison with control muffins made using commercial gluten-free corn flour were evaluated in this study. The proximate chemical composition, antioxidant capacity analysed by ABTS, photochemiluminescence and cyclic voltammetry assays, and inhibitory activity against protein glycation in vitro in BSA/Glu systems were investigated. The content of the total phenolic compounds, available lysine, furosine, free and total FIC, browning index and antioxidant capacity of buckwheat-enhanced gluten-free muffins were higher compared to the control samples. Gluten-free muffins made of the fermented buckwheat flour showed a significantly higher antioxidant capacity, an increased activity against AGEs formation and an increased available lysine content, as well as a higher FAST index and browning index as compared to the muffins obtained with unfermented buckwheat flour. The study showed that buckwheat flour fermented by L. plantarum could be used as an ingredient for improving the functional properties of gluten-free muffins.
Keywords: Buckwheat flour, Fermentation, Lactobacillus plantarum, Gluten-free muffins, Functional properties
Introduction
Muffins are one of the popular sweet snacks known all around the world. People suffering for celiac disease are unable to consume them because they are traditionally prepared from wheat flour with added eggs, sugar, oil or fat and milk, yeasts or baking powder. Nowadays, scientific data is available regarding gluten-free muffins prepared from different types of flours, like chickpea (Herranz et al. 2016), rice (Nozawa et al. 2016; Singh et al. 2015, 2016), corn (Marcet et al. 2015) or buckwheat (Ciesarová et al. 2016). Also protease treatment is used to produce wheat flour with partially hydrolysed gluten that may be used for preparing hypoimmunogenic muffins as presented by Umashankar et al. (2016).
In their review article, Zannini et al. (2012) presented the feasibility of applying microbial fermentation to produce gluten-free products with improved quality. They emphasised how important it is to look for the optimal microbial starter cultures to reengineer gluten-free products and processes. Cereals are good sources of nutrients for a number of species of the Lactobacillus genus (Charalampopoulos et al. 2009; Müller et al. 2001). Fermentation of cereals with lactic acid bacteria, including lactobacilli, may result in many types of metabolites with putative bioactivity like, e.g., cobalamin, reuterin, riboflavin, and γ-aminobutyrate (Russo et al. 2014; Stromeck et al. 2011). Ciesarová et al. (2016) demonstrated that muffins made of unfermented and fermented buckwheat flour suspension showed higher contents of potassium, magnesium, zinc and manganese compared to the control muffins made of gluten-free corn flour.
Buckwheat (Fagopyrum esculentum Moench) has been used as a component of gluten-free products and reported to improve the technological and overall sensory quality of bread (Dapčević Hadnađev et al. 2013; Wronkowska et al. 2013). This pseudocereal is a significant source of rutin, catechins and polyphenols, with their potential antioxidant activity having a significant effect on a nutritional value (Wronkowska et al. 2010). Giménez-Bastida and Zieliński (2015) presented an overview of recent in vitro and in vivo studies concerning health benefits resulting from buckwheat consumption. They pointed to the need for designing future in vitro studies which will allow indicating compounds responsible for the observed beneficial effects, trying to identify the mechanisms which underlie the positive impact on health.
The aim of this study was to evaluate the selected functional properties of four types of gluten-free muffins made of unfermented and fermented, by Lactobacillus plantarum, common buckwheat flour in comparison with muffins made of commercial gluten-free corn flour.
Materials and methods
Ingredients for muffin formulation
The following ingredients were used: commercial granulated sugar (Korunný cukor, Slovenské cukrovary s.r.o., Sereď, Slovakia), sunflower oil (Prommiena, produced for Lidl, Nemšová, Slovakia), eggs (medium size 53–63 g, produced for Lidl, Nemšová, Slovakia), salt (Castello, produced for Lidl, Nemšová, Slovakia), and sodium bicarbonate p.a. (NaHCO3, Slavus, Bratislava, Slovakia). Gluten-free corn flour (containing: corn starch, corn flour, guar gum and dextrose, according to producer’s declaration) was provided by Dr. Schär AG/SPA (Italy). Flour from common buckwheat was provided by the local industry from North–East Poland.
Muffin-making process
The procedure for the preparation of buckwheat or gluten-free corn flour suspensions and for the preparation of a fermented suspension of flour was described in detail by Ciesarová et al. (2016). Briefly, 25 g of buckwheat flour or gluten-free corn flour was mixed with 100 mL of water, boiled (up to thick consistency of the flour suspension) and then sterilised in an autoclave (121 °C for 15 min, at 200 kPa). The fermented buckwheat flour suspension was prepared by mixing the suspension with 1 mL of inoculum of L. plantarum S-lak 1 (collection from Stuvital, Ltd., Slovakia) and then incubation for 24 h at 25 °C, without mixing. The basic formulas of control and experimental muffins are shown in Table 1. The mixture of all ingredients was blended (planetary rotation of mixing) using a 5-speed KitchenAid mixer Model 5KSM150PS (Artisan, USA). The dough (50-g portions) was baked in paper cups at 180 °C for 25 min. Baking tests were carried out in an electric oven (Miwe Condo, Germany). The mass of fresh muffins, after cooling, was circa 40 g. The dry matter (DM) of muffins was determined after 48-h pre-drying at ambient temperature using a moisture analyser IR-120 (Denver Instrument, Germany). Sample abbreviations were presented under Table 1.
Table 1.
Recipe of experimental muffins
| Ingredients (g) | Control | Gluten-free buckwheat | Gluten-free buckwheat/corn | ||
|---|---|---|---|---|---|
| Unfermented UB | Fermented FB | Unfermented UG | Fermented FG | ||
| Buckwheat flour | – | 54 | 54 | – | – |
| Gluten-free corn flour | 54 | – | – | 54 | 54 |
| Gluten-free corn flour in suspension | 36 | – | – | – | – |
| Unfermented buckwheat flour in suspension | – | 36 | – | 36 | – |
| Fermented buckwheat flour in suspension | – | – | 36 | – | 36 |
| Eggs | 116 ± 10 | 116 ± 10 | 116 ± 10 | 116 ± 10 | 116 ± 10 |
| Sugar | 50 | 50 | 50 | 50 | 50 |
| Sunflower oil | 10 | 10 | 10 | 10 | 10 |
| Salt | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| NaHCO3 | 2 | 2 | 2 | 2 | 2 |
Control, control muffins from unfermented gluten-free corn flour suspension with gluten-free corn flour; UB, muffins from unfermented buckwheat flour suspension with buckwheat flour; FB, muffins from fermented buckwheat flour suspension with buckwheat flour; UG, muffins from unfermented buckwheat flour suspension with gluten-free corn flour; FG, muffins from fermented buckwheat flour suspension with gluten-free corn flour
Chemicals and reagents
2,2′-Azinobis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), rutin (quercetin-3-rutinoside), d-glucose, bovine serum albumin (BSA), and lysine (Nα-acetyl-l-lysine) were purchased from Sigma (Sigma Chemical Co., St. Louis, MO, USA). The kit for the photochemiluminescence (PCL) assay was from Analytik Jena AG (Jena, Germany). o-phtaldialdehyde for fluorescence (OPA) and sodium dodecylsulfonate (SDS) were supplied by Fluka (Buchs, Switzerland). Furosine (2-furoylmethyl-lysine) was purchased from PolPeptide (Strasbourg, France). Methanol (HPLC purity) was provided by POCh (Gliwice, Poland). Water was purified with the Mili-Q-system (Milipore, Bedford, USA).
Methods
The protein, ash and fat content of the gluten-free muffins was determined using AOAC (2005) method while total carbohydrate was determined by difference.
Assays of available lysine, furosine, free fluorescence intermediary compounds (FIC), FAST index and browning index were conducted as described by Michalska et al. (2008). Materials for these analyses were prepared as follows: dry samples were mixed with 6% of aqueous SDS, incubated for 30 min with stirring every 10 min for 30 s and filtrated, and then the filtrates were used for the analyses.
The OPA assay was used to determine the content of available lysine (fluorescence at λEx = 340 and λEm = 455 nm). For the quantitative analysis of available lysine, the external standard method was used and values obtained were expressed as FI/mg of dry matter (DM).
Furosine, 2-furoylmethyl-lysine, content was determined with the chromatographic method. The samples were hydrolysed with 8 mL of 8 M HCl at 110 °C for 23 h under anaerobic conditions, and then the hydrolysates were filtrated and used for further analysis. The external standard method was applied for the quantitative analysis of furosine by using of a commercial standard of pure furosine, and data were expressed as milligrams per one grams of DM sample.
The free fluorescence intermediary compounds (FIC) were measured at λEx = 353 and λEm = 438 nm. FIC data were expressed as arbitrary fluorescence intensity per one milligram of DM sample (FI/mg).
The FAST index was calculated according to Birlouez-Aragon et al. (2001), based on the analysis of fluorescence due to advanced MRPs measured at λEx = 353 and λEm = 438 nm and tryptophan fluorescence at λEx = 290 and λEm = 340 nm. Data of FAST index were expressed in percent (w/w).
The formation of brown pigments in the examined muffin samples was estimated according del Castillo et al. (2002). The assay was performed in a Coulter DU 800 spectrophotometer (Beckman Instruments Inc., Fullerton, CA), at the absorbance 420 nm. Results were expressed as arbitrary absorbance units.
For determination of rutin and total phenolic compounds (TPC) content, and for ABTS and PCL assays of antioxidant capacity, extracts from muffins were prepared as follows: 100 mg of muffin powder were extracted with 1 mL of 80% (v/v) methanol, after ultrasonic vibration for 30 s, the solution was mixed and centrifuged at 5000xg at 4 °C for 5 min. That step was repeated 5 times and the supernatants were collected into a 5-mL flask. The final extract concentration was 20 mg/mL.
Rutin content was analysed in an HPLC system (Shimadzu, Kyoto, Japan) consisting of two pumps (LC-10 AD), a UV detector (SPD-10A) set at 330 nm, an autosampler set for 5 μL injection (SIL-10 ADVP), a column oven (CTO-10 ASVP), and a system controller (SIL-10 ADVP) according to the method described by Zielińska et al. (2010).
Total phenolic compounds (TPC) was determined according to Shahidi and Naczk (1995). Muffin extracts (0.25 mL) were mixed with 0.25 mL of the Folin-Ciocalteu reagent, 0.5 mL of Na2CO3 solution and 4 mL of water, the mixture was left for 25 min at room temperature, and then was centrifuged at 2000xg for 10 min. A UV-160 1PC spectrophotometer (Shimadzu, Japan) was used to measure the absorbance at 725 nm. The results were expressed as milligrams of gallic acid equivalents (GAE) per gram of DM.
Antioxidant capacities of 80% aqueous methanol extracts from muffin samples were determined against ABTS+● radical cation using a spectrophotometric assay by Re et al. (1999) with a minor modification. For the photometric assay, 1.48 mL of the ABTS+● solution and 20 μL of muffin extracts or Trolox standards were mixed and measured immediately, and again after 6 min at 30 °C and 734 nm using a UV-160 1PC spectrophotometer (Shimadzu, Kyoto, Japan). Appropriate solvent blanks were run in each assay. The antioxidant capacity was calculated on the basis of percentage inhibition of absorbance at 734 nm using a Trolox standard curve and was expressed as μmol Trolox equivalents (Trolox Eq) per one g of sample DM.
Antioxidant capacities were analysed using the photochemiluminescence (PCL) method described by Popov and Lewin (1999) with PHOTOCHEM® apparatus (Analytik Jena, Leipzig, Germany). The muffin extracts were analysed according to Analytic Jena protocols. The total antioxidant capacity (PCL) was calculated as the sum of the values obtained for lipophilic (PCL-ACL) and hydrophilic (PCL-ACW) extracts of muffins and was expressed as μmol Trolox equivalents (Trolox Eq) per one g of sample DM.
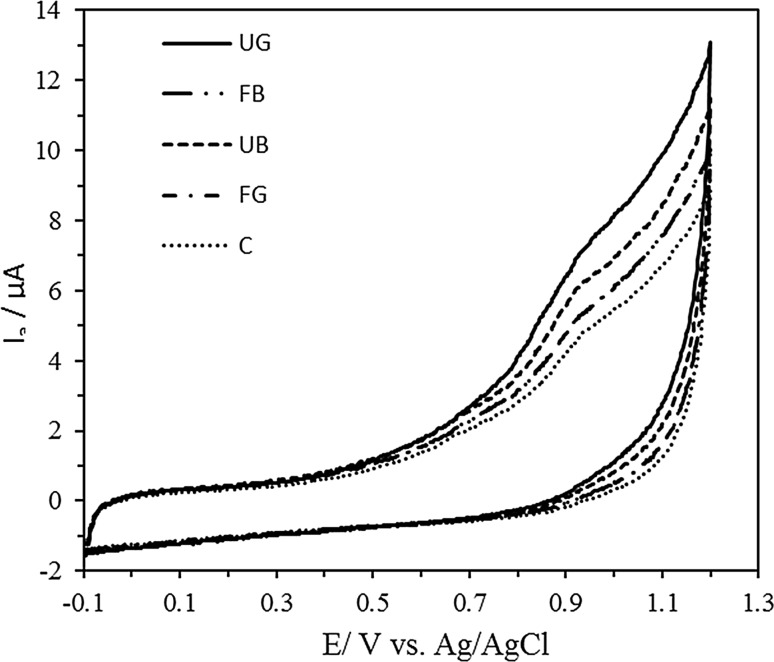
Cyclic voltammetry experiments (CV) were performed in 80% methanol extracts of gluten-free muffins (200 mg/mL) mixed with 0.2 M sodium acetate–acetic buffer (pH 4.5) at the ratio of 1:1 (v/v) according to Zieliński et al. (2012). The sodium acetate–acetic buffer acted also as a supporting electrolyte for cyclic voltammetry measurements. A micro-electrochemical cell (with the total volume of 200 μL), made of Teflon, was used in the experiment. The cell comprised three electrodes: a glassy carbon (GC) working electrode (BAS MF-2012, 3 mm diameter), an Ag/AgCl (3.5 M KCl) reference and a Pt (0.5 mm diameter coiled Pt wire) counter electrode. The cyclic voltammetry experiment were performed in the range of −100 to 1200 mV at a potential sweep-rate of 100 mV s−1 at room temperature using a G 750 potentiostat/galvanostat (Gamry Ins., USA). The higher total charge under anodic current wave indicates a higher reducing capacity of the investigated muffin extracts. The reducing capacity of gluten-free muffins was expressed in terms of µmol Trolox per one g of sample DM.
To analyse the inhibitory activity against protein glycation (AGEs) in vitro in a bovine serum albumin/glucose (BSA/Glu) assay, muffin extracts were prepared as follows: 0.5 g of powdered muffins were extracted with 5 mL of the 80% methanol aqueous solution (40 min, 25 °C) to the final concentration of 50 mg/mL, and then dried. The dried samples were dissolved in 5 mL of a phosphate buffer (0.1 mol/L, pH 7.4) and used directly for the anti-glycation tests as it was described in details by Szawara-Nowak et al. (2014). Fluorescence intensity (excitation wave of 330 nm and emission wave of 410 nm) was measured using an LS 50B luminescent spectrophotometer (Perkin Elmer, USA). Triplicate samples were run for each set and the percent inhibition of AGEs formation by a muffin extract or aminoguanidine solution (1 mmol/L) used as a positive control, was calculated.
Statistical analysis
The measurements were performed in three replications for each type of muffins obtained from two separate baking processes for every formulation. The reported data are the mean results for each formulation with the standard deviation. The results obtained were analysed by one-way ANOVA. Fisher’s Least Significant Difference Test was performed at a significance level of p < 0.05 for post hoc comparison.
Results and discussion
The proximate composition of gluten-free muffins made of fermented and unfermented buckwheat flour is presented in Table 2. The highest protein content was found in the muffins prepared from unfermented and fermented buckwheat flour (UB and FB, respectively). The highest fat content was found in control muffins, whereas the lowest in UB muffins. The gluten-free muffins obtained from unfermented or fermented, by L. plantarum, common buckwheat flour suspension mixed with buckwheat flour showed the highest content of ash compared to the other analysed samples. As presented Ciesarová et al. (2016), buckwheat flour is a better source on macro- and microelements compared to gluten-free corn flour (7.7 and 1.1 g/kg of ash, respectively). The total carbohydrate content was similar in the analysed muffins, with the lowest value determined in FB muffins. The muffins prepared from gluten-free corn flour with unfermented or fermented buckwheat flour (UG and FG, respectively) showed higher protein content as compared to the control samples. It is important from the nutritional point of view, because the amino acid composition of buckwheat proteins is well balanced, they are rich in arginine and lysine being the primary amino acids limiting the content of proteins in cereals (Wronkowska et al. 2010). Also their digestibility was relatively low (Kato et al. 2001), and they exhibited some functional properties such as hypercholesterolaemic activity in rats fed a high-cholesterol diet as reported by Tomotake et al. (2002).
Table 2.
Proximate chemical composition of gluten-free muffins
| Type of muffin | Dry matter (%) | Proteins content (g/100 g DM) | Fats content (g/100 g DM) | Ash content (g/100 g DM) | Carbohydrates content (g/100 g DM) |
|---|---|---|---|---|---|
| Control | 92.8 ± 0.1b | 7.6 ± 0.7c | 11.3 ± 0.1a | 1.2 ± 0.3c | 72.7 ± 0.1b |
| UB | 92.3 ± 0.2b | 10.4 ± 0.9a | 9.8 ± 0.2c | 1.8 ± 0.3a | 70.3 ± 0.6c |
| FB | 95.5 ± 0.1a | 10.7 ± 0.8a | 10.9 ± 0.1b | 1.8 ± 0.3a | 72.1 ± 0.3b |
| UG | 90.7 ± 0.3c | 8.3 ± 0.6c | 10.2 ± 0.1b | 1.6 ± 0.3b | 70.6 ± 0.1c |
| FG | 95.3 ± 0.2a | 9.2 ± 0.3b | 10.4 ± 0.1b | 1.6 ± 0.3b | 74.1 ± 0.2a |
Values are mean ± standard deviation (n = 3). Values in each column with different letters are significantly different (p < 0.05). DM dry matter; UB, FB, UG, FG abbreviation as in Table 1
Buckwheat groats are sources of quercetin and rutin (Kreft et al. 2006). However, muffins produced exclusively from buckwheat flour did not show statistically significant differences in the content of rutin compared to the other muffins (Table 3). The content of total phenolic compounds (TPC) was higher in the muffins prepared from unfermented or fermented buckwheat flour suspension (UB and FB, respectively) compared to the other samples. The fermentation by L. plantarum had no significant effect on TPC contents compared to the muffins made of the unfermented buckwheat suspension. Soong et al. (2014) found that the total phenolics content in the muffins baked with corn was higher compared to the muffins made of oat, wheat, and rice flour, but determined the highest total phenolics content in the muffins from barley flour. As presented by Dordević et al. (2010), fermentation of buckwheat by S. cerevisiae and L. rhamnosus caused TPC increase compared to the non-fermented samples. A significant increase of phenolic acids content in soybean fermented with different microorganisms (Aspergillus oryzae, Rhizopus oryzae and Bacillus subtilis) was also found by Dueñas et al. (2012).
Table 3.
Content of rutin and total phenolic compounds (TPC), and antioxidant capacity of gluten-free muffins analysed with PCL, ABTS and CV methods
| Type of muffin | Rutin (μg/g DM) | TPC (mg GAE/g DM) | Antioxidant capacity (μmol of Trolox Eq/g DM) | ||
|---|---|---|---|---|---|
| PCL | ABTS | CV | |||
| Control | 10.49 ± 0.74 | 21.06 ± 0.54d | 0.40 ± 0.01d | 18.00 ± 1.79c | 0.62 ± 0.22d |
| UB | 11.60 ± 1.02 | 26.28 ± 0.26a | 0.75 ± 0.00b | 22.46 ± 2.60b | 1.45 ± 0.12c |
| FB | 11.81 ± 2.87 | 25.44 ± 0.55ab | 0.97 ± 0.01a | 28.99 ± 0.59a | 1.80 ± 0.28b |
| UG | 11.07 ± 0.97 | 24.78 ± 1.05c | 0.51 ± 0.01c | 20.49 ± 2.05c | 2.29 ± 0.18a |
| FG | 11.11 ± 3.09 | 24.48 ± 0.33c | 0.90 ± 0.00a | 24.20 ± 0.33b | 2.24 ± 0.26a |
Values are mean ± standard deviation (n = 3). Values in each column with different letters are significantly different (p < 0.05). TPC total phenolic acids; GAE gallic acid equivalents; DM dry matter; UB, FB, UG, FG abbreviation as in Table 1
The antioxidant capacity of gluten-free muffins was analysed with PCL, ABTS and CV methods (Table 3, Fig. 1). The muffins made of fermented and unfermented buckwheat flour had the highest antioxidant capacity compared to the control muffins prepared from the unfermented gluten-free corn flour suspension with gluten-free corn flour. It should be noted that the fermented buckwheat flour suspension used in the recipes was significantly increasing the antioxidant capacity of the muffins (FB and FG). As reported by Soong et al. (2014), the antioxidant capacity of corn, wheat and oat muffins was comparable, while barley muffin demonstrated the highest ability to scavenge ABTS and DPPH free radicals. The fermentation process can enhance the level of many bioactive compounds in cereals, but of course the type of fermentation affects the potentially bioactive constituents obtained. Fermentation of buckwheat, wheat germ, barley and rye by lactic acid bacteria or yeast showed that this process led to an increase of antioxidant activities, as presented by Dordević et al. (2010).
Fig. 1.
Cyclic voltammograms of gluten-free muffins. Measurements were performed with 80% methanol extracts (200 mg/mL) mixed with 0.2 M sodium acetate-acetic buffer (pH 4.5) at the ratio of 1:1 (v/v); scan rate 100 mV s−1. Sample description as under Table 1
Table 4 summarises tryptophan content and results obtained in the OPA assay. In the control samples, prepared from the unfermented gluten-free corn flour suspension with gluten-free corn flour, the content of tryptophan was the highest (7.3 FI/mg DM). Available lysine content in the control samples was lower (3.1 mg/g DM) compared to other analysed muffins. The fermentation procedure used to prepare the fermented buckwheat flour suspension had a beneficial effect since the available lysine content was higher compared to the muffins with the unfermented buckwheat flour suspension. Cereal grains, like corn, have low lysine and tryptophan content as compared to buckwheat (Arendt and Zannini 2013). Bilgiçli (2009) showed that 40% addition of buckwheat flour to prepare of tarhana (traditional Turkish fermented cereal food) significantly increased lysine content.
Table 4.
Tryptophan, available lysine and Maillard reaction products (MRPs) in gluten-free muffins
| Type of muffin | Tryptophan (FI/mg DM) | Available lysine (mg/g DM) | Early MRPs | Advanced MRPs | Final MRPs | ||
|---|---|---|---|---|---|---|---|
| Furosine (mg/g DM) | Free FIC (FI/mg DM) | Total FIC (FI/mg DM) | FAST index (%) | Browning index (AU) | |||
| Control | 7.3 ± 0.38a | 3.07 ± 0.14d | 0.37 ± 0.08d | 4.5 ± 0.27d | 32.11 ± 0.14c | 61.64d | 0.096 ± 0.009b |
| UB | 5.8 ± 0.61c | 4.32 ± 0.22b | 1.32 ± 0.09a | 12.6 ± 1.08b | 84.52 ± 7.05b | 217.24b | 0.098 ± 0.008b |
| FB | 6.1 ± 0.15bc | 5.52 ± 0.38a | 0.99 ± 0.04b | 15.4 ± 1.04a | 90.22 ± 6.72b | 252.46a | 0.168 ± 0.016a |
| UG | 6.9 ± 1.00ab | 3.38 ± 0.33 cd | 0.81 ± 0.04c | 11.1 ± 0.96bc | 118.44 ± 11.49a | 160.87c | 0.167 ± 0.013a |
| FG | 5.3 ± 0.25c | 3.80 ± 0.53bc | 0.70 ± 0.03c | 10.8 ± 0.66c | 118.71 ± 2.58a | 203.77b | 0.198 ± 0.026a |
Values are mean ± standard deviation (n = 3). Values in each column with different small superscript letters are significantly different (p ≤ 0.05). FI fluorescence intensity; AU arbitrary units; UB, FB, UG, FG abbreviation as in Table 1
Contents of the early, advanced and final Maillard reaction products in gluten-free muffins are shown in Table 4. Furosine is one of the markers of the early stage of the Maillard reaction. In our study, the production of furosine was observed in all types of muffins. Its lowest content was found in control muffins (0.4 mg/g DM). Muffins prepared exclusively from buckwheat flour, UB and FB, had the highest content of furosine (1.32 and 0.99 mg/g DM, respectively). For both muffins with the fermented buckwheat flour suspension (FB and FG), a decrease of the furosine content (not significant for FG) was observed compared to the muffins with the unfermented buckwheat flour suspension.
Advanced MRPs analyses in this study included: free and total fluorescence of the intermediary compounds (FIC), also the FAST index was calculated. The content of Free FIC content of muffins prepared exclusively from buckwheat flour (UB and FB) was about 2.5-times higher compared to the control sample, and it was higher compared to UG and FG muffins. The opposite situation was found during the determination of the total FIC, the highest content was found for UG and FG as compared to UB and FB and it was about three times higher compared to the control muffins. These findings indicate that UG and FG muffins contained higher content of linked-to-protein fluorescent compounds as compared to muffins prepared from buckwheat flour (UB and FB). The lowest level of linked-to-protein fluorescent compounds was found in control sample.
The FAST index, calculated as a ratio of FIC to tryptophan fluorescence, is important from the nutritional point of view for the food produced by heat treatment. The significant increase of FAST index was observed for all investigated muffins compared to the control sample. The UG and FG muffins, prepared from unfermented or fermented buckwheat flour suspension with gluten-free corn flour, showed lower values of the FAST index compared to the UB and FB muffins, which could indicate better nutritional quality of the latter. That index was used by Damjanovic Desic and Birlouez-Aragon (2011) as a sensitive indicator which provides reliable information on the nutritional damage induced by heat treatment in, e.g. infant formulas. Thermal conditions used for rye bread baking caused the increase of FIC and FAST index, for bread crust, as demonstrated by Michalska et al. (2008).
Browning index is used as an indicator of the formation of melanoidin during the final stage of Maillard reaction (Table 4). Muffins prepared from unfermented and fermented buckwheat flour suspension with gluten-free corn flour (UG and FG, respectively) showed higher browning index compared to the muffins prepared exclusively from buckwheat flour (UB and FB). The fermentation process used caused browning index increase in both types of muffins (FB and FG), but not statistically significant for the FG sample. Proteins and reducing sugar could be released from the matrix during the fermentation process. Then after baking process used to produce gluten-free muffins, higher values of the browning index were noticed for muffins FB and FG compared to UB and UG muffins due to the final stage of Maillard reaction. Przygodzka et al. (2015) demonstrated an increase of the browning index in rye-buckwheat cake with the selected spices compared to cakes without spices. Slight formation of brown polymer MRP took place during long-term storage (5 years) of ginger cakes, as presented by Zieliński et al. (2012).
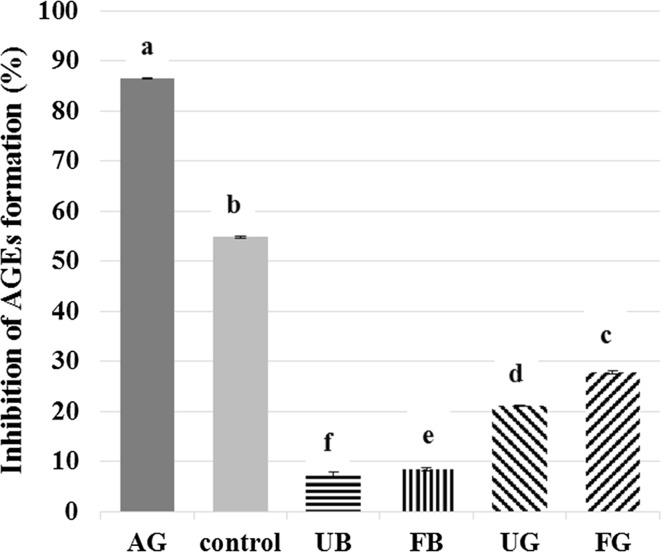
The inhibitory effects of the extracts from buckwheat gluten-free muffins evaluated using the BSA-glucose model are depicted in Fig. 2. The aminoguanidine (AG) solution (1 mmol/L) was used as a reference inhibitor of the glycation process (Zuwało-Jagiełło 2009). AG (a hydrazine compound) prevents AGEs formation by trapping intermediates at the initial glycation stages (Thornalley 2003). It was found that in the BSA-glucose model the inhibitory activity of extracts from muffins prepared from unfermented and fermented buckwheat flour suspension with buckwheat flour (UB and FB, respectively) showed a six-fold lower value as compared to the activity of control muffins from unfermented gluten-free corn flour suspension with gluten-free corn flour. Whereas for extracts from muffins made of the unfermented or fermented buckwheat flour suspension with gluten-free corn flour (UG and FG, respectively) this difference was only about two-fold compared to the control muffin. The inhibitory effect of AG was high and reached 87%. It is noteworthy that the use of the buckwheat flour suspension in the muffins prepared from gluten-free corn flour (UG and FG) affected the improvement of the inhibitory effect of these muffins against AGEs formation in the BSA/glucose model system. Another interesting observation is that the fermentation process applied affected the increase in the degree of inhibition of FB and FG muffins against AGEs formation compared to the muffins prepared from unfermented buckwheat flour suspension (UB and UG). Szawara-Nowak et al. (2014) showed for the buckwheat-enhanced wheat bread that the inhibitory effect against AGEs formation in BSA-glucose system depended on the level of buckwheat substitution and type of wheat flour used. Przygodzka and Zieliński (2015) made a similar observation for rye-buckwheat ginger cakes enriched with rutin. They found that the addition of buckwheat flours as well as rutin supplementation in ginger cakes caused an increase of AGEs inhibitory potential. Also these authors showed that the cakes produced with dough fermentation step had a lower inhibitory activity compared to those without any fermentation step.
Fig. 2.
The inhibitory effects of gluten-free muffins against AGEs formation in the BSA/glucose model system (sample description as under Table 1; AG aminoguanidine)
Conclusion
The proximate chemical composition, antioxidant capacity of gluten-free muffin extracts against ABTS●+ and O●−2radicals (by ABTS and PCL assays) and their inhibitory activity against protein glycation in vitro in BSA/Glu systems were investigated. The increase of protein content in the muffins with the use of buckwheat flour (UG and FG) compared to the control samples is important from the nutritional point of view because the amino acid composition of buckwheat proteins is well balanced. No statistically significant differences were observed in the content of rutin in various muffins. The content of total phenolic compounds (TPC), available lysine, furosine, free and total FIC, browning index and antioxidant capacity of the muffins were higher compared to the control samples. The fermentation process used for buckwheat flour suspension significantly increased the antioxidant capacity, available lysine content, FAST index, browning index and the degree of inhibition against AGEs formation compared to the samples with unfermented buckwheat flour suspension. The fermentation of buckwheat flour suspension by L. plantarum represents a novel technological solution, which could lead to the production of innovative functional products.
Acknowledgements
This work was performed under the Research and Development Cooperation between Slovakia and Poland (Project No. SK-PL-0100-12) and it was funded in part by the statutory funds of the Department of Chemistry and Biodynamics of Food of the IAR&FR PAS. The infrastructure used for experiments was financially supported by the ERDF through implementation of the Projects Nos. ITMS 26240120041 and 26240120042. We cordially thank Mrs. Janka Koreňová from NPPC VUP Bratislava for preparation of inocula and fermentation of buckwheat flour.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- AOAC . Official methods of analysis of AOAC International. 18. Gaithersburg: AOAC International; 2005. [Google Scholar]
- Arendt EK, Zannini E. Chapter 2. Maize. In: Arendt EK, editor. Cereal grains for the food and beverages industries. Cambridge: Woodhead Publishing; 2013. pp. 67–115. [Google Scholar]
- Bilgiçli N. Effect of buckwheat flour on chemical and functional properties of tarhana. LWT Food Sci Technol. 2009;42:514–518. doi: 10.1016/j.lwt.2008.09.006. [DOI] [Google Scholar]
- Birlouez-Aragon I, Leclere J, Quedraogo CL, Birlouez E, Grongnet J-F. The FAST method, a rapid approach of the nutritional quality of heat-treated foods. Nahrung/Food. 2001;45(3):201–205. doi: 10.1002/1521-3803(20010601)45:3<201::AID-FOOD201>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Charalampopoulos D, Vazquez JA, Pandiella SS. Modelling and validation of Lactobacillus plantarum fermentations in cereal-based media with different sugar concentrations and buffering capacities. Biochem Eng J. 2009;44(2):96–105. doi: 10.1016/j.bej.2008.11.004. [DOI] [Google Scholar]
- Ciesarová Z, Basil E, Kukurová K, Marková L, Zieliński H, Wronkowska M. Gluten-free muffins based on fermented and unfermented buckwheat flour—content of selected elements. J Food Nutr Res. 2016;55:108–113. [Google Scholar]
- Damjanovic Desic S, Birlouez-Aragon I. The FAST index—a highly sensitive indicator of the heat impact on infant formula model. Food Chem. 2011;124:1043–1049. doi: 10.1016/j.foodchem.2010.07.071. [DOI] [Google Scholar]
- Dapčević Hadnađev TR, Torbica AM, Hadnađev MS. Influence of buckwheat flour and carboxymethyl cellulose on rheological behaviour and baking performance of gluten-free cookie dough. Food Bioprocess Technol. 2013;6:1770–1781. doi: 10.1007/s11947-012-0841-6. [DOI] [Google Scholar]
- del Castillo MD, Ames JM, Gordon MH. Effect of roasting on the antioxidant activity of coffee brews. J Agr Food Chem. 2002;50:3698–3703. doi: 10.1021/jf011702q. [DOI] [PubMed] [Google Scholar]
- Dordević TM, Šiler-Marinković SS, Dimitrijević-Branković SI. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010;119:957–963. doi: 10.1016/j.foodchem.2009.07.049. [DOI] [Google Scholar]
- Dueñas M, Hernandez T, Robredo S, Lamparski G, Estrella I, Munoz R. Bioactive phenolic compounds of soybean (Glycine max cv. Merit): modifications by different microbiological fermentations. Pol J Food Nutr Sci. 2012;62:241–250. [Google Scholar]
- Giménez-Bastida JA, Zieliński H. Buckwheat as a functional food and its effects on health. J Agr Food Chem. 2015;63:7896–7913. doi: 10.1021/acs.jafc.5b02498. [DOI] [PubMed] [Google Scholar]
- Herranz B, Canet W, Jiménez MJ, Fuentes R, Alvarez MD. Characterisation of chickpea flour-based gluten-free batters and muffins with added biopolymers: rheological, physical and sensory properties. Food Sci Technol. 2016;51:1087–1098. [Google Scholar]
- Kato N, Kayashita J, Tomotake H. Nutritional and physiological functions of buckwheat protein. Recent Res Dev Nutr. 2001;4:113–119. [Google Scholar]
- Kreft I, Fabjan N, Yasumoto K. Rutin content in buckwheat (Fagopyrum esculentum Moench) food materials and products. Food Chem. 2006;98:508–512. doi: 10.1016/j.foodchem.2005.05.081. [DOI] [Google Scholar]
- Marcet I, Paredes B, Díaz M. Egg yolk granules as low-cholesterol replacer of whole egg yolk in the preparation of gluten-free muffins. LWT Food Sci Technol. 2015;62:613–619. doi: 10.1016/j.lwt.2014.08.031. [DOI] [Google Scholar]
- Michalska A, Amigo-Benavent M, Zielinski H, del Castillo MD. Effect of bread making on formation of Maillard reaction products contributing to the overall antioxidant activity of rye bread. J Cereal Sci. 2008;48:123–132. doi: 10.1016/j.jcs.2007.08.012. [DOI] [Google Scholar]
- Müller MR, Wolfrum G, Stolz P, Ehrmann MA, Vogel RF. Monitoring the growth of Lactobacillus species during a rye flour fermentation. Food Microbiol. 2001;18(2):217–227. doi: 10.1006/fmic.2000.0394. [DOI] [Google Scholar]
- Nozawa M, Ito S, Arai E. Effect of ovoalbumin on the quality of gluten-free rice flour bread made with soymilk. LWT Food Sci Technol. 2016;66:598–605. doi: 10.1016/j.lwt.2015.11.010. [DOI] [Google Scholar]
- Popov I, Lewin G. Antioxidative homeostasis: characterisation by means of chemiluminescence technique in methods in enzymology. In: Packer L, editor. Oxidants and antioxidants, Part B. London: Academic Press; 1999. pp. 96–100. [Google Scholar]
- Przygodzka M, Zieliński H. Evaluation of in vitro inhibitory activity of rye-buckwheat ginger cakes with rutin on the formation of advanced glycation end-products (AGEs) Pol J Food Nutr Sci. 2015;65:191–198. [Google Scholar]
- Przygodzka M, Zieliński H, Ciesarová Z, Kukurová K, Lamparski G. Study on sensory quality, antioxidant properties, and Maillard reaction products formation in rye-buckwheat cakes enhanced with selected spices. J Chem. 2015;418639:9. [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1237–1291. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Russo P, Capozzi V, Arena MP, Spadaccino G, Dueñas MT, López P, Fiocco D, Spano G. Riboflavin-overproducing strains of Lactobacillus fermentum for riboflavin-enriched bread. Appl Microbiol Biotechnol. 2014;98(8):3691–3700. doi: 10.1007/s00253-013-5484-7. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Naczk M. Methods of analysis and quantification of phenolic compounds. In: Shahidi F, Naczk M, editors. Food phenolic: sources, chemistry, effects and applications. Lancaster: Technomic Publishing Company; 1995. pp. 287–293. [Google Scholar]
- Singh JP, Kaur A, Shevkani K, Singh N. Influence of jambolan (Syzygium cumini) and xanthan gum incorporation on the physicochemical, antioxidant and sensory properties of gluten-free eggless rice muffins. Int J Food Sci Technol. 2015;50:1190–1197. doi: 10.1111/ijfs.12764. [DOI] [Google Scholar]
- Singh JP, Kaur A, Singh N. Development of eggless gluten-free rice muffins utilizing black carrot dietary fibre concentrate and xanthan gum. J Food Sci Technol. 2016;53(2):1269–1278. doi: 10.1007/s13197-015-2103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong YY, Tan SP, Leong LP, Henry JK. Total antioxidant capacity and starch digestibility of muffins baked with rice, wheat, oat, corn and barley flour. Food Chem. 2014;164:462–469. doi: 10.1016/j.foodchem.2014.05.041. [DOI] [PubMed] [Google Scholar]
- Stromeck A, Hu Y, Chen L, Gänzle MG. Proteolysis and bioconversion of cereal proteins to glutamate and γ-aminobutyrate (GABA) in rye malt sourdoughs. J Agr Food Chem. 2011;59(4):1392–1399. doi: 10.1021/jf103546t. [DOI] [PubMed] [Google Scholar]
- Szawara-Nowak D, Koutsidis G, Wiczkowski W, Zieliński H. Evaluation of the in vitro inhibitory effects of buckwheat enhanced wheat bread extracts on the formation of advanced glycation end-products (AGEs) LWT Food Sci Technol. 2014;58:327–334. doi: 10.1016/j.lwt.2013.03.005. [DOI] [Google Scholar]
- Thornalley PJ. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation end products. Arch Biochem Biophys. 2003;419:31–40. doi: 10.1016/j.abb.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Tomotake H, Shimaoka I, Kayashita J, Nakajoh M, Kato N. Physicochemical and functional properties of buckwheat protein product. J Agr Food Chem. 2002;50:2125–2129. doi: 10.1021/jf011248q. [DOI] [PubMed] [Google Scholar]
- Umashankar AK, Rajiv J, Prabhasankar P. Development of hypoimmunogenic muffins: batter rheology, quality characteristics, microstructure and immunochemical validation. Food Sci Technol. 2016;53(1):531–540. doi: 10.1007/s13197-015-2028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wronkowska M, Soral-Śmietana M, Krupa-Kozak U. Buckwheat, as a food component of a high nutritional value, used in the prophylaxis of gastrointestinal diseases. Eur J Plant Sci Biotechnol. 2010;4(special issue 1):64–70. [Google Scholar]
- Wronkowska M, Haros M, Soral-Śmietana M. Effect of starch substitution by buckwheat flour on gluten-free bread quality. Food Bioprocess Technol. 2013;6:1820–1827. doi: 10.1007/s11947-012-0839-0. [DOI] [Google Scholar]
- Zannini E, Pontonio E, Waters DM, Arendt EK. Applications of microbial fermentations for production of gluten-free products and perspectives. Appl Microbiol Biotechnol. 2012;93:473–485. doi: 10.1007/s00253-011-3707-3. [DOI] [PubMed] [Google Scholar]
- Zielińska D, Szawara-Nowak D, Zieliński H. Determination of the antioxidant activity of rutin and its contribution to the antioxidant capacity of diversified buckwheat origin material by updated analytical strategies. Pol J Food Nutr Sci. 2010;60(4):315–321. [Google Scholar]
- Zieliński H, del Castillo MD, Przygodzka M, Ciesarová Z, Kukurová K, Zielińska D. Changes in chemical composition and antioxidative properties of rye ginger cakes during their shelf-life. Food Chem. 2012;135:2965–2973. doi: 10.1016/j.foodchem.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Zuwało-Jagiełło J. Therapeutic intervention in diseases with advanced glycation end products in their pathogenesis. Pol Merk Lek. 2009;27:152–158. [PubMed] [Google Scholar]