Abstract
Commercialization of agricultural products, including seeds and its derived products, represents an important economic source for developing countries. Natural colorants obtained from the seeds of achiote plant (annatto) have been used since pre-Hispanic times. Also, production of this crop has been important for Mayan cuisine. Annual world production of achiote seeds is approximately 14,500 tons (dry weight). Two thirds of the production is commercialized as dried seeds and the rest as colorant. Latin America produces 60% of the total world production, followed by Africa (27%) and Asia (12%). The main producers in Latin America are Peru, Brazil and Mexico. The purpose of the present paper is to review the most recent literature on Bixa orellana L. focusing on bixin, norbixin, tocotrienols and tocopherols biosynthesis, use and industrial applications of annatto extracts, as well as its nutraceutical potential and its benefits for human health.
Keywords: Annatto extract, Bixin, Norbixin, Tocotrienols, Tocopherols
Introduction
Achiote (Bixa orellana L.) is a plant native to tropical America, probably to the Amazon basin in Brazil. It is widely cultivated in all tropical regions around the world; specifically in Colombia, Mexico, Ecuador and in the Peruvian Andes. In Mexico, 40 accessions of achiote have been collected and morphologically characterized; in addition, for 20 of these accessions its biochemical characterization, only in terms of bixin content, has been determined.
An accession is a sample of a distinct germplasm, where germplasm is defined as the set of genes expressed in the distinctive phenotype; therefore, collection of different accessions and its preservation in germplasm banks is very important for conservation of genetic diversity and its potential commercial usage (Fig. 1b).
Fig. 1.
a Plant of achiote (Bixa orellana L.), b biological diversity, c fruits and d Seeds
Achiote is a perennial tree that can reach nine meters of height with white or pink flowers and fruits with the form of globular ovoid capsules (Fig. 1a, c). Each capsule may contain from 30 to 45 ovoid or cone-shaped seeds covered by a thin red–orange layer or viscous aril (Fonnegra and Jiménez 2007, Fig. 1d). Native Americans have known for long achiote’s uses and value, mainly as natural color in food products and as body/facial paint. Achiote is also known as achote, achote of the mountain, achioti, bixa, bija, bijol, bijo, urucú, onoto, annatto orellana, orlean, pumacua, bicha, caituco, chacangaricua, among other names (Janick 2012). The name of ‘achiote’ derives from the Nahuatl word ‘achiotl’, which means shiny seed, and its species scientific name was given in honor to its discoverer, Francisco Orellana (Morton 1960).
Achiote (Bixa orellana L.) as a natural pigment source
Annatto is the name given to the crude pigment extract (containing bixin, norbixin and other carotenoids in different proportions) obtained from achiote. While bixin is fat soluble, norbixin is hydrosoluble. The possibility for obtaining water-soluble colorants as well as oil-soluble colorants depending on the type of extraction as well as the solvent and temperature used has converted achiote in one of the most interesting plant sources of vegetable colorants (Smith 2006).
Natural and synthetic colorants
Currently, the use of artificial colorants such as tartrazine (E102), allura red (E129) or sunset yellow FCF (E110) in food products have been severely questioned in developed countries, since there are reports showing that indiscriminate consumption of these colorants is associated to the development of degenerative illnesses such as some types of cancer (Salinas et al. 2005). As a consequence, the use of some artificial food colors such as carmoisine (E122) and Ponceau 4R (E124) have been banned in the USA and Europe and the use of natural colorants such as the dye extracted from the surface of B. orellana L. (E 160b, annatto extract) have been recommended (McCann 2007).
Nowadays, annatto extract has a great economic importance worldwide and it is one of the natural colors more widely used in the food, cosmetic and pharmaceutical industries, because it does not alter flavor and it is practically not toxic (Michelangeli et al. 2002; Smith 2006; Lourido and Martínez 2010).
This natural dye is rich on the carotenoids bixin (dark red color) and norbixin or orelline (yellow color) which are mainly used to develop attractive colors in dairy products (cheeses, margarine, and butter), meats, ice creams, cosmetics, condiments, ceramic, paint, hair colors, soaps, nail polish, varnish, lacquer, fabric colors, among others (Devia and Saldarriaga 2003). Bixin represents approximately 80% of the total carotenoids content present in the dye obtained from achiote (mainly in its 9-cis configuration with low amounts of trans-bixin and cis-norbixin) (Smith 2006). Other apocarotenoids present in lower amounts are norbixin, bixin dimethyl ester and byproducts of lycopene degradation (Cardarelli et al. 2008).
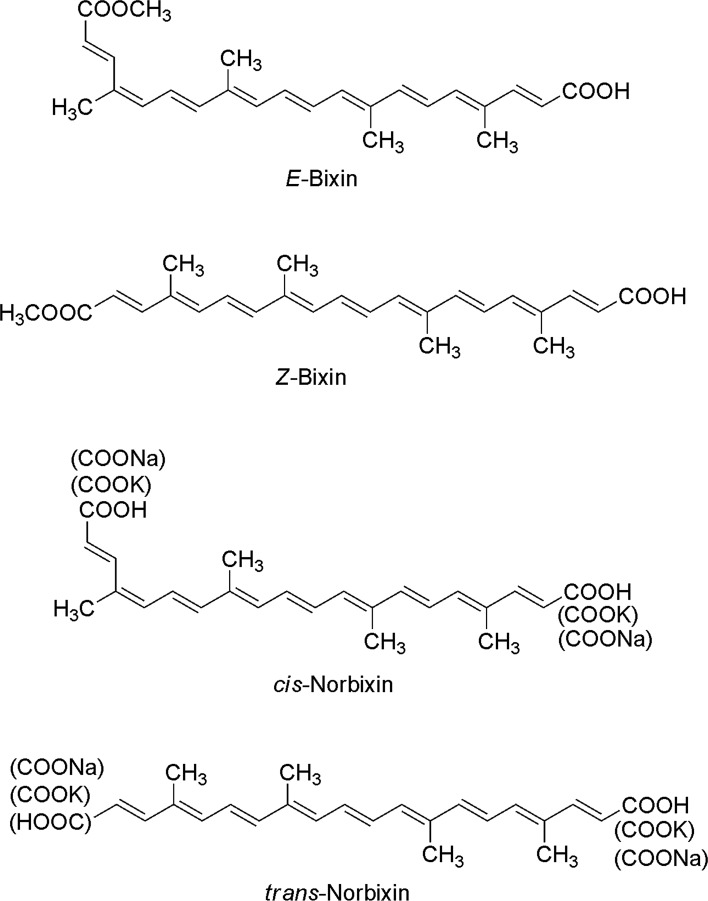
Bixin is the only carotenoid that presents a 9-cis configuration (structure present in an oxygenated carotenoid, lutein, which belongs to the xanthophyll group) and 2 carboxyl groups (Fig. 2).
Fig. 2.
The structural formulae of bixin and norbixina (modified from Smith 2006)
Bixin, norbixin, tocotrienols and tocopherols biosynthesis
Carotenoids biosynthesis
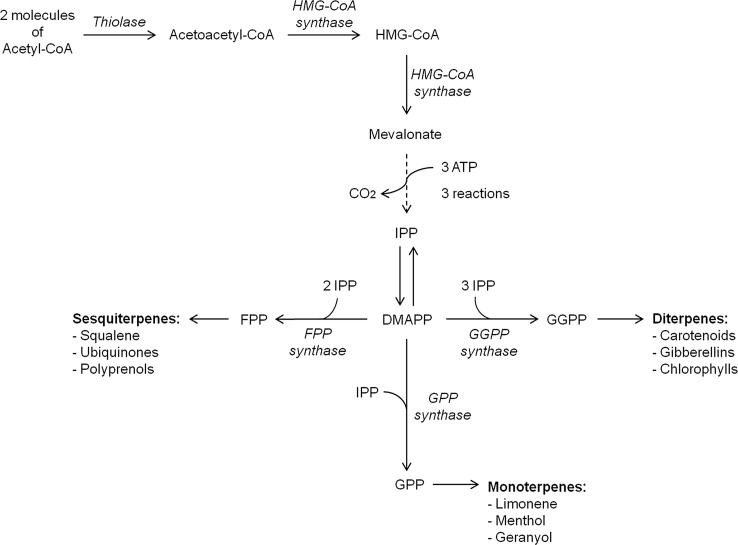
In order to understand bixin and norbixin biosynthetic pathway, it is important to know the main steps for carotenoids biosynthesis. Carotenoids belong to the terpene family, compounds formed by isoprene units, and their biosynthesis is through the isoprenoid pathway (Schmidt et al. 2010, Fig. 3).
Fig. 3.
Pathway of biosynthesis of isoprenoid compounds. Abbreviations HMG-CoA β-hydroxy-β-methylglutaryl-coenzyme A, ATP adenosine triphosphate, IPP isopentenyl diphosphate, DMAPP dimethylallyl diphosphate, FPP farnesyl diphosphate, GGPP geranylgeranyl diphosphate, GPP geranyl diphosphate (modified from Schmidt et al. 2010)
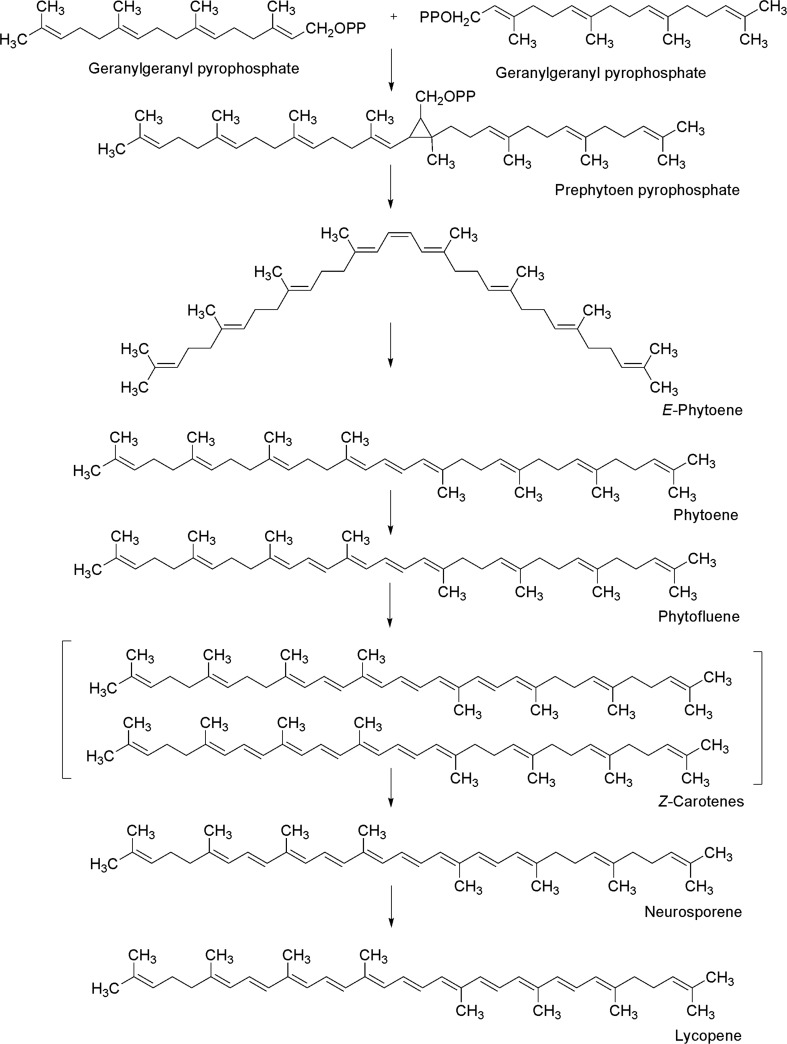
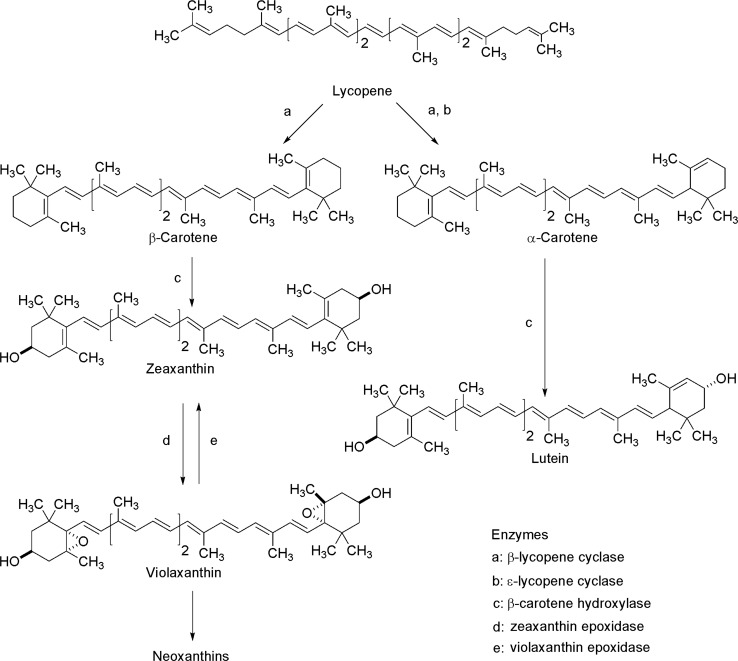
The first step for carotenoids biosynthesis is the condensation of two molecules of geranylgeranyl pyrophosphate (GGPP) to produce cis-phytoene (C40), the basic structure of all carotenoids. Subsequently, cis-phytoene suffers four desaturation reactions for the synthesis of lycopene (Delgado-Vargas et al. 2000, Fig. 4). Lycopene is the simplest carotenoid molecule (linear molecule formed of 40 carbon atoms) and is the substrate for generating most cyclic carotenoids through cycling, hydroxylation or oxidation or the combination of these three types of reaction. These reactions take place on one of end of the lycopene chain; in this way the biosynthetic pathway branches to form the distinct carotenoids (Castro and Murcia 2013, Fig. 5).
Fig. 4.
Lycopene biosynthesis (modified from Delgado-Vargas et al. 2000)
Fig. 5.
Formation of carotenoids from lycopene (modified from Delgado-Vargas et al. 2000)
Bixin and norbixin biosynthesis
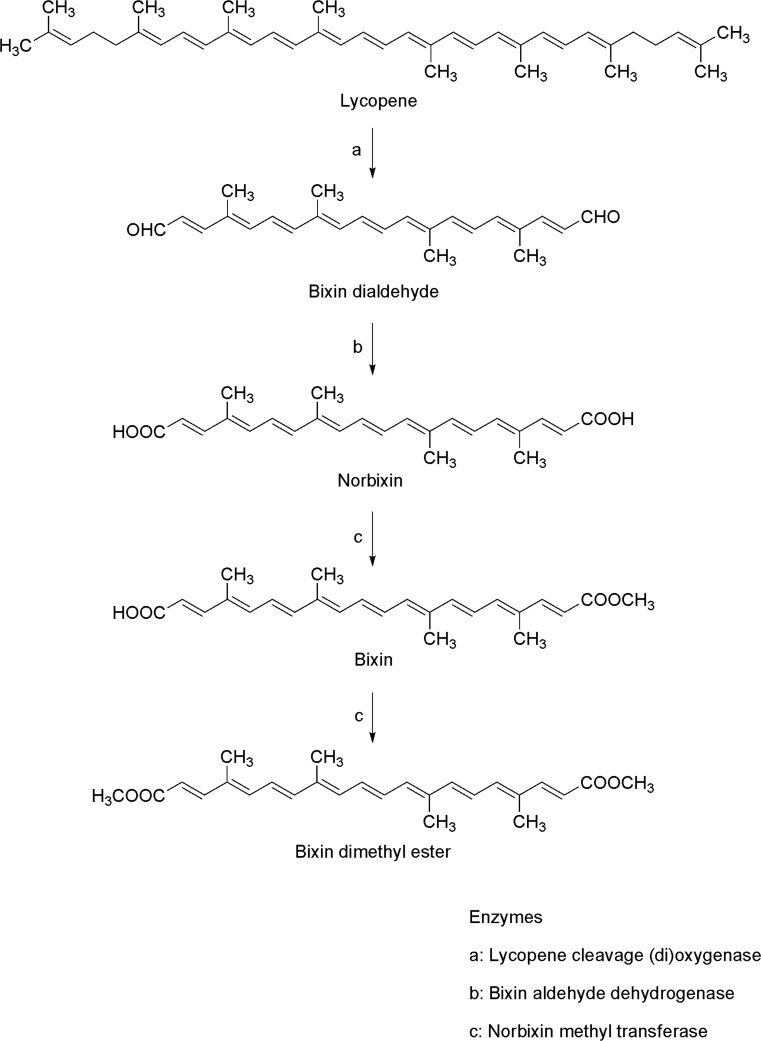
It has been reported that lycopene is also the possible substrate for bixin and norbixin biosynthesis that occurs through the cleavage of the 5–6 and 5′–6′ double bonds on the ends of lycopene linear chain (which structure is stereochemically similar to bixin) through the action of lycopene cleavage (di)oxygenase, followed by oxidation of bixin dialdehyde catalyzed by bixin aldehyde dehydrogenase and finally, by transference of a methyl group to the carboxyl end group through the action of norbixin methyl transferase (Zaldívar and Godoy 2003; Giuliano et al. 2003; Siva et al. 2010, Fig. 6).
Fig. 6.
Proposed bixin biosynthesis pathway. All compounds are shown in their all-trans configuration (modified from Giuliano et al. 2003)
Tocopherols and tocotrienols biosynthesis
On another hand, it has been documented that achiote (B. orellana L.) contains important amounts of tocotrienols, tocopherols, terpenes and flavonoids both in the seeds and in the leaves (Rodrigues et al. 2007; Chisté et al. 2011).
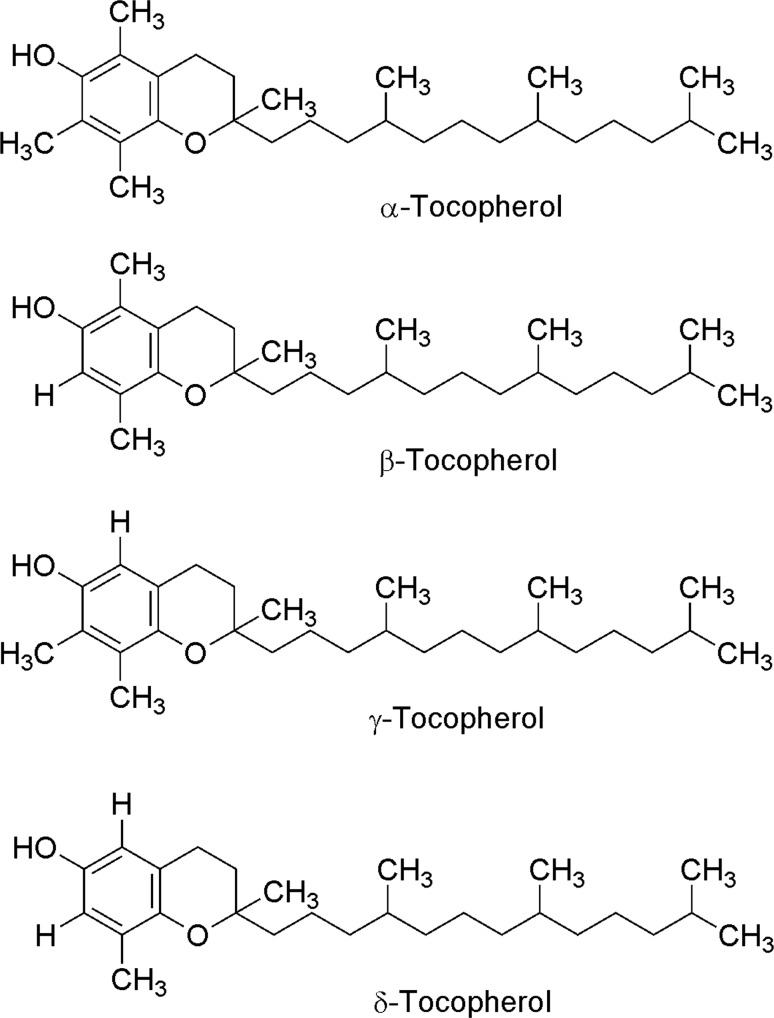
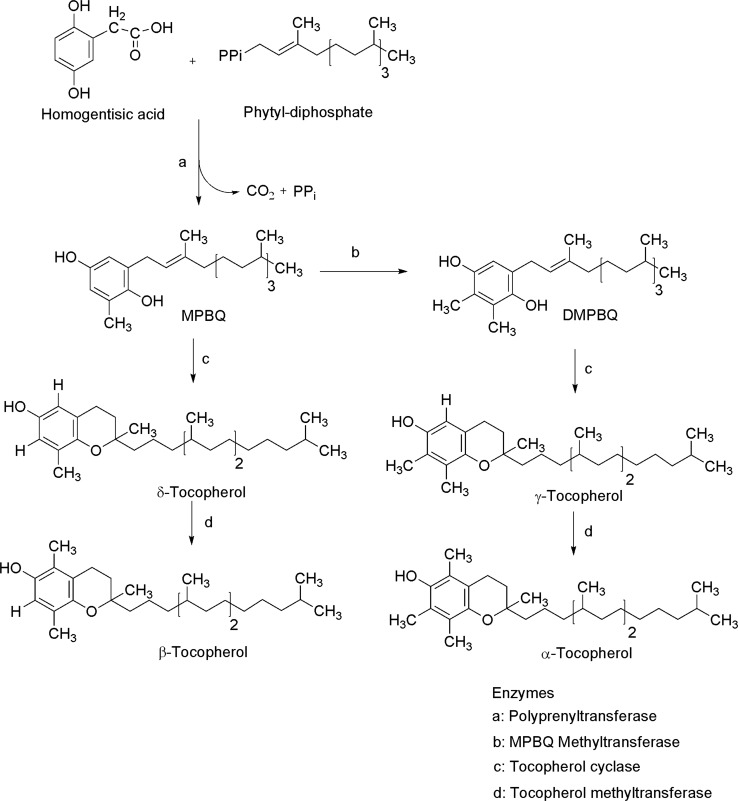
Vitamin E is the generic name given to all compounds that exerts the biological functions of α-tocoferol (Frank et al. 2012). However, vitamin E is a family of lipophilic compounds which includes four tocopherols and four tocotrienols (α, β, γ, δ) (Aggarwal et al. 2010, Figs. 7, 8). The structure of these two types of compounds is very similar; but, while tocopherols have a saturated phytol tail, tocotrienols possess an isoprenoid tail with three unsaturated residues (Das et al. 2007; Almeida et al. 2011). Tocopherols and tocotrienols biosynthesis takes place in the chloroplasts from the aromatic ring of the homogentisic acid. Tocopherols synthesis starts with condensation of homogentisic acid and phytyl diphosphate to form 2-methyl-6-phytyl-1,4-benzoquinol (MPBQ). Afterwards, cycling reaction for chromanol ring formation generating δ-tocopherol and β-tocopherol. On the other hand, γ-tocoferol and α-tocoferol are synthesized through the action of tocopherol cyclase and γ-tocopherol methyltransferase, respectively (Fig. 9).
Fig. 7.
Chemical structure of tocopherols (modified from Aggarwal et al. 2010)
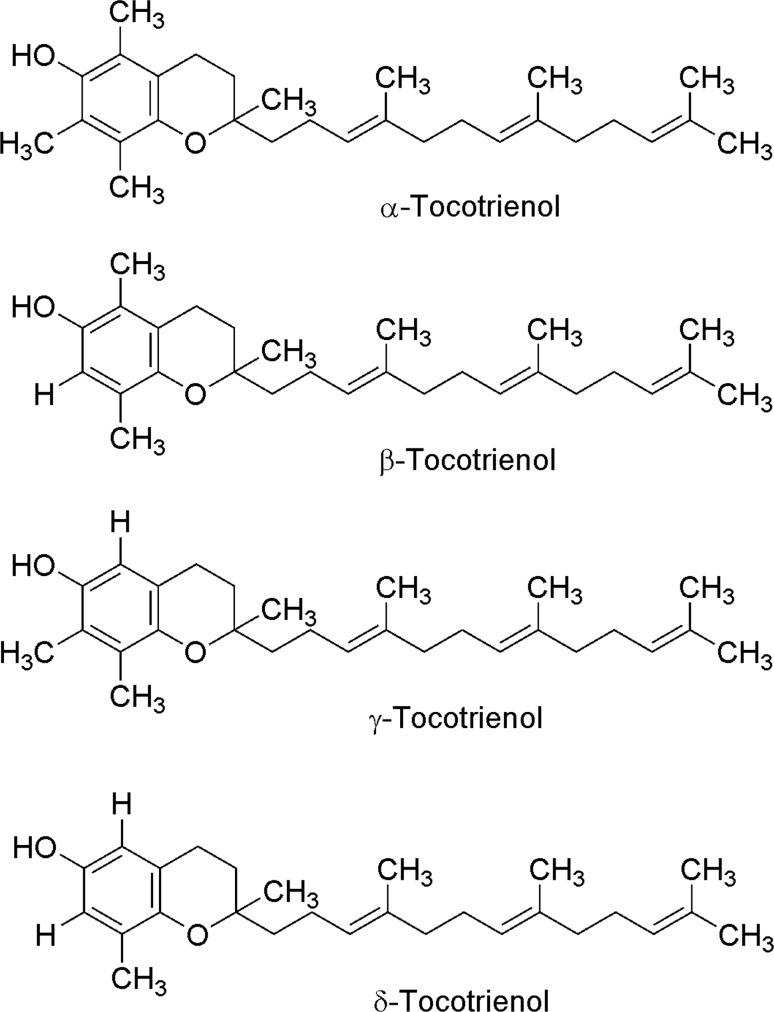
Fig. 8.
Chemical structure of tocotrienols (modified from Aggarwal et al. 2010)
Fig. 9.
Tocopherols biosynthesis (modified from Eitenmiller and Lee 2004)
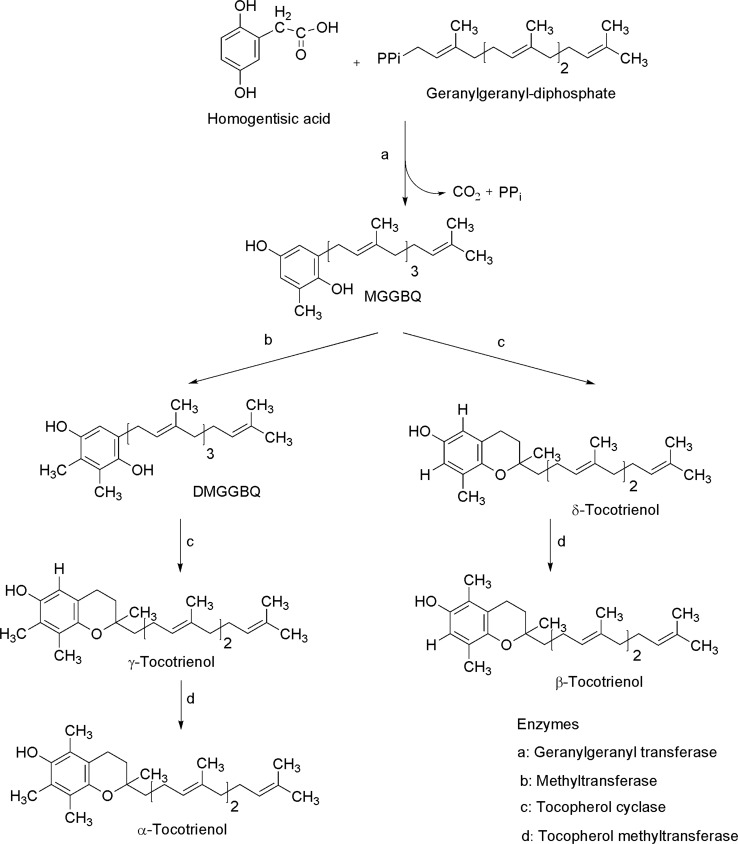
Tocotrienols synthesis starts with condensation of homogentisic acid and geranylgeranyl diphosphate to generate 2-methyl-6-geranylgeranyl-1,4-benzoquinol (MGGBQ) catalyzed by geranylgeranyl transferase. γ-tocotrienol and α-tocotrienol are synthesized from 2,3-dimethyl-6-geranylgeranyl-1,4-benzoquinol (DMGGBQ). Moreover, δ-tocotrienol and β-tocotrienol are synthesized from MGGBQ through the action of tocopherol cyclase and γ-tocopherol methyltransferase, respectively (Eitenmiller and Lee 2004; Almeida et al. 2011, Fig. 10).
Fig. 10.
Tocotrienols biosynthesis (modified from Eitenmiller and Lee 2004)
Use and industrial applications of achiote extracts
Achiote seeds and the extracts obtained from them have been used for more than 200 years in Europe and North America (for giving a red–orange–yellow coloring to food products (Smith 2006). Nowadays, bixin occupies the second place among the main natural colors used worldwide (Lauro and Francis 2000; Giridhar and Parimalan 2010; Chuyen et al. 2012).
There are several forms of the extract from achiote (more than any other natural color) available for its application on the food and pharmaceutical industries (Hendry and Houghton 1992) and depends on the nature of the extract which food products are colored (Smith 2006).
Types of extracts (commercial forms of the dye)
In most of achiote producing countries, the ground seed is commercialized as condiment or colorant for food; whereas in developed countries it is mainly used in the form of extracts used as a natural color in the food industry. International commercialization of these extracts is regulated by the quality standards of each country where is commercialized. Annatto extracts may contain different proportions of bixin and norbixin, depending on the extraction process and the temperature used. The different proportions of these compounds determine the tone and hue of the food product that is colored (Smith 2006). Annatto extracts are widely used in the food industry, mainly in dairy and other food products such as poultry, fish, surimi (imitation of crab meat), bakery, breakfast cereals, several desserts, jam and jellies, maraschino cherries, cream and icing for cakes, sauces, condiments, candies and beverages such as aperitifs, soft-drinks and juices.
Oil soluble annatto extract
Soybean, grapeseed and sunflower oils can be used to extract or dissolve bixin. The oil soluble extract contains mainly cis-bixin. This extract shows different range of color tonalities from yellow to orange-reddish. If bixin is extracted with hot vegetable oil, there is a change in the color that goes from orange–reddish to orange–yellow (Smith 2006) and also, cis-bixin is transformed in trans-bixin which is its most stable and soluble form (Hendry and Houghton 1992). If the bixin solution is heated for longer periods of time, the trans-bixin is converted to thermal degradation compounds called “Yellow C-17” for its characteristic yellow color. Annatto extracts in oil generally contains between 0.05 and 1.0% of bixin; higher concentrations are impossible to obtain because bixin is hardly soluble in oil (Smith 2006). Even though the coloring power of these solutions is low, they are used to color dairy products (cream-cheese and American cheese), salad dressings and some aperitifs (Hendry and Houghton 1992). They are also used to dye wood, leather, lacquers, fabrics and paints (Lourido and Martínez 2010) as well as diverse products of the cosmetic industry (Giuliano et al. 2003).
Oil suspension
Unlike annatto extract in oil, bixin content in the suspension is approximately 4%, being cis-bixin and trans-bixin the main compounds present in the extract. For preparing the suspension, annatto dye powder is suspended and/or dissolved in vegetable oil. Bixin oil suspensions show tonalities of the orange color provided that the product temperature does not increase above 100 °C; if the temperature is higher the end product will be yellow (Hendry and Houghton 1992).
Water soluble extract
Water soluble annatto extract consists of the dissociated form of norbixin in an alkaline solution, usually potassium or sodium hydroxide and then acidified with hydrochloric acid for precipitating norbixin. The precipitate is filtered, dried and ground for obtaining a granular powder which is commercialized as norbixin powder or in diluted liquid solutions (Smith 2006). Norbixin, particularly in its cis form, is very soluble in water and hence, these solutions may contain from 0.1 to 5% of this pigment. Water soluble norbixin powders generally contain between 1 and 15% of the pigment. The use of water soluble annatto extract has considerably increased, not only in the dairy industry, but also in aperitifs and beverages processing (Hendry and Houghton 1992). Dairy products represent one of the most important categories of processed food with annatto extracts. Traditionally, cheese has been colored with norbixin solutions for more than 200 years since one of the most important factors for cheese coloration is the interaction and union of the proteins of the milk with the annatto dye, ensuring a permanent coloration (Emerton 2008). Hydrosoluble achiote extract is an excellent colorant; it has been reported that 1 L of 1% norbixin solution is enough for coloring 16 tons of cheese (Giuliano et al. 2003).
Emulsion
Emulsified annatto contains bixin and/or norbixin associated with and emulsifier such as propylene glycol and polisorbates (Hendry and Houghton 1992). The addition of an emulsifier to the bixin and/or norbixin extract makes it miscible for food products that contain both and aqueous phase and an oil phase. Some examples are ice cream, margarine, mayonnaise, creamy sauces, some candies, and wide range of bakery products. Emulsified annatto pigment is commercialized as a liquid and contains between 1 and 2.5% of bixin or norbixina (Smith 2006).
Regulation of annatto extract as natural pigment
The daily human intake of annatto extract is an important consideration from the regulatory standpoint. Based on the amount of annatto used in diverse processed food products, many calculations have been made in order to determine the estimated daily human consumption of bixin/norbixin. The estimates varies from 0.032 mg/kg body weight/day of bixin and 0.051 mg/kg body weight/day of norbixin in the United States, to 0.065 mg/kg body weight/day of bixin in the United Kingdom. Calculated for a person of 60 kg average of body weight, these figures represent and average daily intake of bixin/norbixin of 1.9 mg of bixin and 3.1 mg of norbixin in the United States and 3.9 of bixin in the United Kingdom (Lauro and Francis 2000).
The maximum acceptable daily intake (ADI) established by Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO) Expert Committee on Food Additives (JECFA 1982) is of 0.065 mg/kg body weight/day of bixin. These calculations have become more important since it was reported that bixin is rapidly absorbed to the blood stream after its ingestion. The human ingestion of a single dose of 16 mg of bixin induced a maximum level of 144 µg/L of bixin/norbixin in the plasma, which is comparable to the range of other carotenoids normally found in the human plasma (β-carotene, 40–530 µg/L; lutein, 90–140 µg/L and lycopene, 70–460 µg/L). Complete bixin and norbixin removal from the plasma occurred after 8 and 24 h of ingestion, respectively (Lauro and Francis 2000).
Annatto is considered as a non-toxic colorant. In the United States annatto is a natural colorant exempted from certification. Its safety is based on the traditional and quotidian use of achiote for more than 200 years as a food color and condiment by millions of people in South America. Furthermore, several authors have performed studies on genotoxicity and chronic oral toxicity in rats and dogs fed for a year with annatto extracted with water and solvents. Researchers concluded that high levels of bixin and norbixin in the diet of the experimental animals did not cause any toxic or carcinogenic effect and that 0.065 mg/kg body weight/day of bixin is an acceptable intake for humans (Haveland-Smith 1981; Ghorpade et al. 1995; Lauro and Francis 2000). Occasionally, allergic reactions to food products containing annatto have been reported, but they have been attributed to protein traces of the achiote seed found as impurities in the colorant (Lauro and Francis 2000).
Potential nutraceutical and health benefits
Chemical composition of Bixa orellana
As mentioned earlier, B. orellana seeds are rich in carotenoids, especially apocarotenes like bixin, isobixin and norbixina (Cardarelli et al. 2008). Other carotenoids present in minor proportions are β-carotene, cryptoxanthin, lutein, zeaxanthin, methylbixin, six apocarotenoids (C30 and C32), eight diapocarotenoids (C19, C22, C24 and C25) and a carotenoid derivative (C14). Moreover, B.a orellana has been described as the vegetable source with the highest content of terpenes such as E-geranylgeraniol, which can reach 57% of total terpenes in the dry seed. Other isoprenoids that have been reported are farnesylacetone, geranylgeranyl octadecanoate and geranylgeranyl formate. It also contains lipids (linoleic acid and in smaller proportions α-linolenic and oleic acids), aminoacids (glutamate, aspartate, leucine) and a great amount of phosphorus, iron, and zinc (Lourido and Martínez 2010).
Phytochemical studies of achiote leaf have revealed the presence of bixaghanene, bixein, bixol, crocetin, ellagic acid, isobixin, phenylalanine, salicylic acid, threonine, tomentosic acid, tryptophan, flavonoid bisulfates, sterols, tannins, saponins, and an esencial oil mainly comprised by sesquiterpenes being ishwarane the major compound. Presence of the triterpene tomentosic acid has also been reported in the roots (Venugopalan et al. 2011).
Tocotrienols and tocopherols
Tocotrienols and tocopherols (α, β, γ, δ) are stereoisomers of vitamin E, which is fat soluble and act as an antioxidant (Patacsil et al. 2012). Vitamin E is recognized by the biological activity of α-tocopherol, but it has been observed that the family of tocotrienols and tocopherols show other functions different to α-tocopherol. Tocopherols and tocotrienols have a similar chemical structure, a chromanol ring with a hydroxyl group that can donate a hydrogen atom for reducing free radicals. The difference between tocopherols and tocotrienols is the presence of three double bonds in the lateral hydrophobic chain of tocotrienols (Srivastava and Gupta 2006). While tocopherols are present in seeds and leaves of most plants, tocotrienols are only present in a small fraction of them (Patacsil et al. 2012). Some of the main sources with the highest tocotrienols/tocopherols ratio are rice bran oil (50/50), palm (75/25) and achiote seeds (99.9/0.1), the last ones being the vegetable sources with the highest amount of tocotrienols (Aggarwal et al. 2010). It has been suggested that these compounds act as antioxidants by protecting the lipid membrane from oxidative damage (lipid peroxidation) (Aggarwal et al. 2010; Frank et al. 2012; Lee et al. 2012; Quadrana et al. 2013). While tocopherols have been widely studied for their antioxidant properties, there are few studies of tocotrienols on this sense. Recent studies have shown that tocotrienols possess a strong anti-carcinogenic and neuroprotective effect as well also involved in reducing cholesterol levels as compared to tocopherols (Sen et al. 2007; Aggarwal et al. 2010; Miyazawa et al. 2011; Wilankar et al. 2011). It has been proposed that these beneficial effects might be due to the unsaturation of their aliphatic tail which makes easier their penetration to tissues and confers them a higher antioxidant power (Samarjit et al. 2007).
Bixa orellana in folk medicine
South American indigenous people have traditionally used annatto extracts from leaves, roots and seeds for medicinal purposes like treating diarrhea and asthma (Lauro and Francis 2000). The aril covering the seed is widely used to treat burns and bleeding, dysentery, gonorrhea, constipation and fever. There are also reports on the use of B. orellana leaves, bark and roots extracts as antidotes for poisoning with Manihot esculenta Crantz, Jatropha curcas L., and Hura crepitans L. In Brazil, annatto extracts are used for treating heartburn, as diuretic and laxative. Decoction of the leaves is used for stopping vomit, nausea and heartburn, for treating urinary and prostate disorders, hypertension, high cholesterol, cystitis, obesity, renal insufficiency, for eliminating uric acid, as well as for several stomach problems.
Piura tribe of the Amazon Rainforest prepare a tea with young roots as aphrodisiac, astringent, and to treat skin problems, fever, dysentery and hepatitis. In Colombia, annatto is used as antivenin for snakebites and the seeds are used as expectorant and for treating gonorrhea (Venugopalan et al. 2011).
Other medicinal properties attributed to achiote extracts both from leaves and seeds are antitumoral (specifically for mouth cancer), anti-inflammatory, emollient, antiseptic, antibacterial, antioxidant, cicatrizant, hypoglycemic activity, as source of vitamins, for treating acne, alopecia, condyloma, measles, smallpox, bacterial infections, conjunctivitis, dermatosis, diabetes, epilepsy, glaucoma, headaches, hemorrhoids, hepatic disorders, malaria, nephrosis, stomachache, stomatitis, tonsillitis and for wound healing and burns (Lourido and Martínez 2010).
Our studies and perspectives
During the last years, efforts have been made to recover achiote cultivation in the Yucatan Peninsula, where the production of this crop has been important for Mayan cuisine through time. However, nowadays there are few commercial plantations; most achiote is cultivated in backyards. In addition, there is not a germplasm bank to guarantee homogeneity of the seeds that are used. For this reason, in 2008 the Achiote Network of the National System of Phytogenetic resources for food and agriculture (SINAREFI for its initials in Spanish) was created to study the situation of achiote genetic resources in Mexico. This network is formed by a multidisciplinary group of enthusiastic researchers from different government and academic institutions (INIFAP, UACH, CICY, IT CONKAL and UAM-Iztapalapa). Work has been done to recover germplasm cultivated by producers in the States of Yucatan, Campeche and Quintana Roo. From this collection work, 40 accessions were obtained; these accessions have been protected and propagated to characterize them in terms of their bixin and norbixin content. In this sense, our group in the laboratory of Plant Physiology and Molecular Biology has focused on the biochemical characterization of these accession specifically on the quantification of the yield of the pigment extraction, color parameters, levels of tocotrienols, tocopherols, luteolin and apigenin, antioxidant capacity in the annatto extracts and its effect on in vivo models.
Also, according to the strategic plan 2015–2018 of the Achiote Network, our group expects to carry out studies for optimizing the process for obtaining the colorant and using the waste products for elaboration of several food products derived from achiote. Furthermore, it has been also proposed research on the use of the aerial parts of the achiote plant and their possible pharmacological properties and health benefits; adding economical value to achiote crop production.
Conclusion
Color is a very important physical attribute in foodstuffs and can modify consumer’s preference. Currently, natural colorants are worldwide favored because a segment of the market demands ‘all natural’ products. Although achiote (B. orellana) is an economically important crop currently remains underutilized. Annatto extract is a natural colorant widely accepted and used in the food industry. The most common industrial way for obtaining the annatto extract is using a KOH alkaline solution. However, the yield of this process for getting this natural dye is only 6% of the processed tissue, i.e. 6 kg of colorant per ton of tissue. This review also pretends to explore the biosynthesis pathway of other important compounds such as tocopherols and tocotrienols in the achiote besides the dyes present.
Acknowledgements
This review is dedicated to Universidad Autónoma Metropolitana—Iztapalapa, SINAREFI—RED ACHIOTE, and PROMEP, who have supported several research projects to characterize, preserve and propagate the genetic material of achiote (Bixa orellana L.). We thank the CONACYT for their financial support (No. 233307) to Denise Raddatz Mota during her Ph.D. studies. This work is a part of D. Raddatz Ph.D. dissertation. We also thank IT Conkal and CICY for assistance with achiote seed sampling.
References
- Aggarwal BB, Sundaram C, Prasad S, Kannappan R. Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem Pharmacol. 2010;80:1613–1631. doi: 10.1016/j.bcp.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida J, Quadrana L, Asís R, Setta N, Godoy F, Bermúdez L, Otaiza NS, Corréa da Silva JV, Fernie AR, Carrari F, Rossi M. Genetic dissection of vitamin E biosynthesis in tomato. J Exp Bot. 2011;62:3781–3798. doi: 10.1093/jxb/err055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardarelli CR, Benassi MT, Mercadante AZ. Characterization of different annatto extracts based on antioxidant and colour properties. LWT-Food Sci Technol. 2008;41:1689–1693. doi: 10.1016/j.lwt.2007.10.013. [DOI] [Google Scholar]
- Castro CV, Murcia SCL. Licopeno y Salud Humana. Rev Reciteia. 2013;12:42–79. [Google Scholar]
- Chisté RC, Mercadante AZ, Gomes A, Fernandes E, Lima JLFC, Bragagnolo N. In vitro scavenging capacity of annatto seed extracts against reactive oxygen and nitrogen species. Food Chem. 2011;127:419–426. doi: 10.1016/j.foodchem.2010.12.139. [DOI] [PubMed] [Google Scholar]
- Chuyen HV, Hoi NT, Eun JB. Improvement of bixin extraction yield and extraction quality from annatto seed by modification and combination of different extraction methods. Int J Food Sci Tech. 2012;47:1333–1338. doi: 10.1111/j.1365-2621.2012.02977.x. [DOI] [Google Scholar]
- Das S, Kalanithi N, Dipak D. Tocotrienols in cardioprotection. Vitam Horm. 2007;75:285–299. doi: 10.1016/S0083-6729(06)75011-7. [DOI] [PubMed] [Google Scholar]
- Delgado-Vargas F, Jiménez AR, Paredes-López O. Natural pigments: carotenoids, anthocyanins, and betalains—characteristics, biosynthesis, processing, and stability. Crit Rev Food Sci Nutr. 2000;40:173–289. doi: 10.1080/10408690091189257. [DOI] [PubMed] [Google Scholar]
- Devia PJE, Saldarriaga CL. Planta piloto para obtener colorante de la semilla del achiote (Bixa orellana) Rev Univ EAFIT. 2003;39:8–22. [Google Scholar]
- Eitenmiller RR, Lee J (2004) Vitamin E: food chemistry, composition, and analysis. CRC press, New York
- Emerton V (2008) Food colours. Blackwell Publishing, Oxford
- Evaluation of Certain Food Additives and Contaminants Twenty-six report of the joint FAO/WHO expert committee on food additives. World Health Organ Tech Rep Ser. 1982;683:7–51. [PubMed] [Google Scholar]
- Fonnegra GR, Jiménez RSL (2007) Plantas medicinales aprobadas en Colombia. Editorial Universidad de Antioquia, Medellín
- Frank J, Dawn CXW, Schrader C, Eckert GP, Rimbach G. Do tocotrienols have potential as neuroprotective dietary factors? Ageing Res Rev. 2012;11:163–180. doi: 10.1016/j.arr.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Ghorpade VM, Desphande SS, Salunkhe DK (1995) Food additive toxicology. CRC press, New York
- Giridhar P, Parimalan RA. A biotechnological perspective towards improvement of annatto color production for value addition—the influence of biotic elicitors. Asia Pac J Mol Biol Biotechnol. 2010;18:77–79. [Google Scholar]
- Giuliano G, Rosati C, Bramley PM. To dye or not to dye: biochemistry of annatto unveiled. Trends Biotechnol. 2003;21:513–516. doi: 10.1016/j.tibtech.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Haveland-Smith RB. Evaluation of the genotoxicity of some natural food colours using bacterial assays. Mutat Res. 1981;91:285–290. doi: 10.1016/0165-7992(81)90002-6. [DOI] [PubMed] [Google Scholar]
- Hendry GAF, Houghton J (1992) Natural food colorants. Springer US, Glasgow
- Janick J (2012) Horticult Rev 39. United States
- Lauro G, Francis J (2000) Natural food colorants: science and technology. CRC press, New York
- Lee YY, Park HM, Lee CK, Kim SL, Hwang TY, Choi MS, Kwon YU, Kim WH, Kim SJ, Lee SC, Kim YH. Comparing extraction methods for the determination of tocopherols and tocotrienols in seeds and germinating seeds of soybean transformed with OsHGGT. J Food Compost Anal. 2012;27:70–80. doi: 10.1016/j.jfca.2012.03.010. [DOI] [Google Scholar]
- Lourido PH, Martínez SG. La Bixa orellana L. en el tratamiento de afecciones estomatológicas, un tema aún por estudiar. Rev Cubana Farm. 2010;44:231–244. [Google Scholar]
- McCann D. Los aditivos alimentarios y el comportamiento hiperactivo en 3 años y 9.8 años de edad, niños de la comunidad: un ensayo aleatorizado, doble ciego, controlado con placebo. Lancet. 2007;370:1560–1567. doi: 10.1016/S0140-6736(07)61306-3. [DOI] [PubMed] [Google Scholar]
- Michelangeli C, Maureen MA, Artioli P, Mata J. Microsporogénesis y microgametogénesis de onoto (Bixa orellana L.) Acta Cient Venez. 2002;53:171–175. [PubMed] [Google Scholar]
- Miyazawa T, Nakagawa K, Sookwong P. Health benefits of vitamin E in grains, cereals and green vegetables. Trends Food Sci Tech. 2011;22:651–654. doi: 10.1016/j.tifs.2011.07.004. [DOI] [Google Scholar]
- Morton J. Can annatto (Bixa orellana L.), an old source of food color, meet new needs for safe dye? Proc Fla State Hort Soc. 1960;73:301–309. [Google Scholar]
- Patacsil D, Tran AT, Cho SY, Suy S, Saenz F, Malyukova I, Ressom H, Collins SP, Clarke R, Kumar D. Gamma-tocotrienol induced apoptosis is associated with unfolded protein response in human breast cancer cells. J Nutr Biochem. 2012;23:93–100. doi: 10.1016/j.jnutbio.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadrana L, Almeida J, Otaiza SN, Duffy T, Corréa da Silva JV, Godoy F, Asís R, Bermúdez L, Fernie AR, Carrari F, Rossi M. Transcriptional regulation of tocopherol biosynthesis in tomato. Plant Mol Biol. 2013;81:309–325. doi: 10.1007/s11103-012-0001-4. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Soares VLF, Oliveira TM, Gesteira AS, Otoni WC, Costa MGC. Isolation and purification of RNA from tissues rich in polyphenols, polysaccharides, and pigments of annatto (Bixa orellana L.) Mol Biotech. 2007;37:220–224. doi: 10.1007/s12033-007-0070-9. [DOI] [PubMed] [Google Scholar]
- Salinas MY, Rubio HD, Díaz VA. Extracción y uso de pigmentos del grano de maíz (Zea mays) como colorantes en yogur. Arch Latinoam Nutr. 2005;55:293–298. [PubMed] [Google Scholar]
- Samarjit D, Kalanithi N, Dipak KD. Tocotrienols in cardioprotection. Vitam Horm. 2007;75:285–299. doi: 10.1016/S0083-6729(06)75011-7. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Wächtler B, Temp U, Krekling T, Séguin A, Gershenzon JA. Bifunctional geranyl and geranylgeranyl diphosphate synthase is involved in terpene oleoresin formation in Picea abies. Plant Physiol. 2010;152:639–655. doi: 10.1104/pp.109.144691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen CK, Khanna S, Roy S. Tocotrienols in health and disease: the other half of the natural vitamin E family. Mol Asp Med. 2007;28:692–728. doi: 10.1016/j.mam.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siva R, Febin PD, Kunal K, Satyanarayanab VSV, Vijaya K. Molecular characterization of bixin—an important industrial product. Ind Crops Prod. 2010;32:48–53. doi: 10.1016/j.indcrop.2010.03.001. [DOI] [Google Scholar]
- Smith J (2006) Annatto extracts-chemical and technical assessment. Chem Tech Assess Manual 1–21
- Srivastava JK, Gupta S. Tocotrienol-rich fraction of palm oil induces cell cycle arrest and apoptosis selectively in human prostate cancer cells. Biochem Biophys Res Commun. 2006;346:447–453. doi: 10.1016/j.bbrc.2006.05.147. [DOI] [PubMed] [Google Scholar]
- Venugopalan A, Giridhar P, Ravishankar A. Food, ethanobotanical and diversified applications of Bixa orellana L.: a scope for its improvement through biotechnological mediation. Ind J Fund Appl Life Sci. 2011;1:9–31. [Google Scholar]
- Wilankar C, Sharma D, Checker R, Khan NM, Patwardhan R, Patil A, Sandur KS, Devasagayam TPA. Role of immunoregulatory transcription factors in differential immunomodulatory effects of tocotrienoles. Free Radic Biol Med. 2011;51:129–143. doi: 10.1016/j.freeradbiomed.2011.03.038. [DOI] [PubMed] [Google Scholar]
- Zaldívar CJM, Godoy HG. El licopeno es el sustrato para la biosíntesis de bixina. Rev Educ Bioquím. 2003;22:146–154. [Google Scholar]