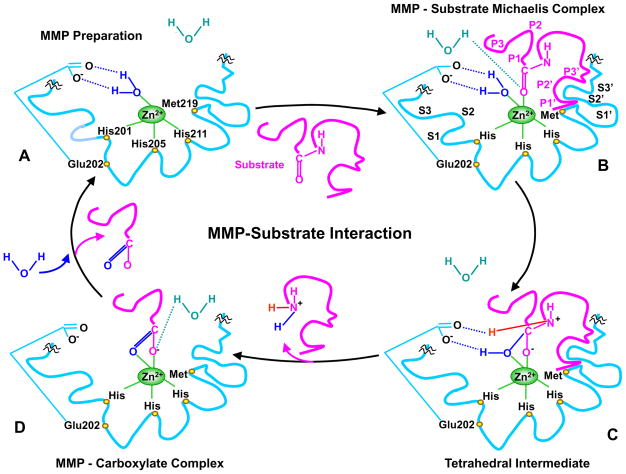
Fig. 2.
MMP-substrate interaction. MMP-3 is used as an example, and slight variations in the MMP-substrate-interaction and the positions of the conserved His and Glu may occur with other MMPs. Only the MMP catalytic domain is illustrated, and the remaining part of the MMP molecule is truncated by squiggles. A) In the quiescent MMP molecule, the catalytic Zn2+ is supported in the HEXXHXXGXXH-motif by binding to the imidazole rings of the 3 histidines His201, 205, 211. Additionally, the methionine-219 (Met219) in the conserved XBMX Met-turn acts as a hydrophobic base to further support the structure surrounding the catalytic Zn2+. In preparation of MMP for substrate binding, an incoming H2O molecule is polarized between the MMP acidic Zn2+ and basic glutamate-202 (Glu202). B) Using H+ from free H2O, the substrate carbonyl group binds to Zn2+, forming a Michaelis complex. This allows the MMP S1, S2, S3, …Sn pockets on the right side of Zn2+ and the primed S1′, S2′, S3′, …Sn′ pockets on the left side of Zn2+ to confer specific binding to the substrate P1, P2, P3, … Pn and the primed P1′, P2′, P3′, … Pn′ substituents, respectively. The MMP pockets are organized such that the S1 and S3 pockets are located away from the catalytic Zn2+, while the S2 pocket is closer to Zn2+. C) The substrate-bound H2O is freed, the Zn2+-bound O from the Glu-bound H2O executes a nucleophilic attack on the substrate carbon, and the Glu202 extracts a proton from the Glu-bound H2O to form an N-H bond with the substrate N, resulting in a tetrahedral intermediate. D) Freed H2O is taken up again, and the second proton from Glu-bound H2O is transferred to the substrate, forming an additional N-H bond. As a result, the substrate scissile C-N bond breaks, thus releasing the N portion of the substrate while the carboxylate portion of the substrate remains in an MMP-carboxylate complex. Another H2O is taken up, thus releasing the remaining carboxylate portion of the substrate, and the MMP is prepared to attack another substrate (A).