Abstract
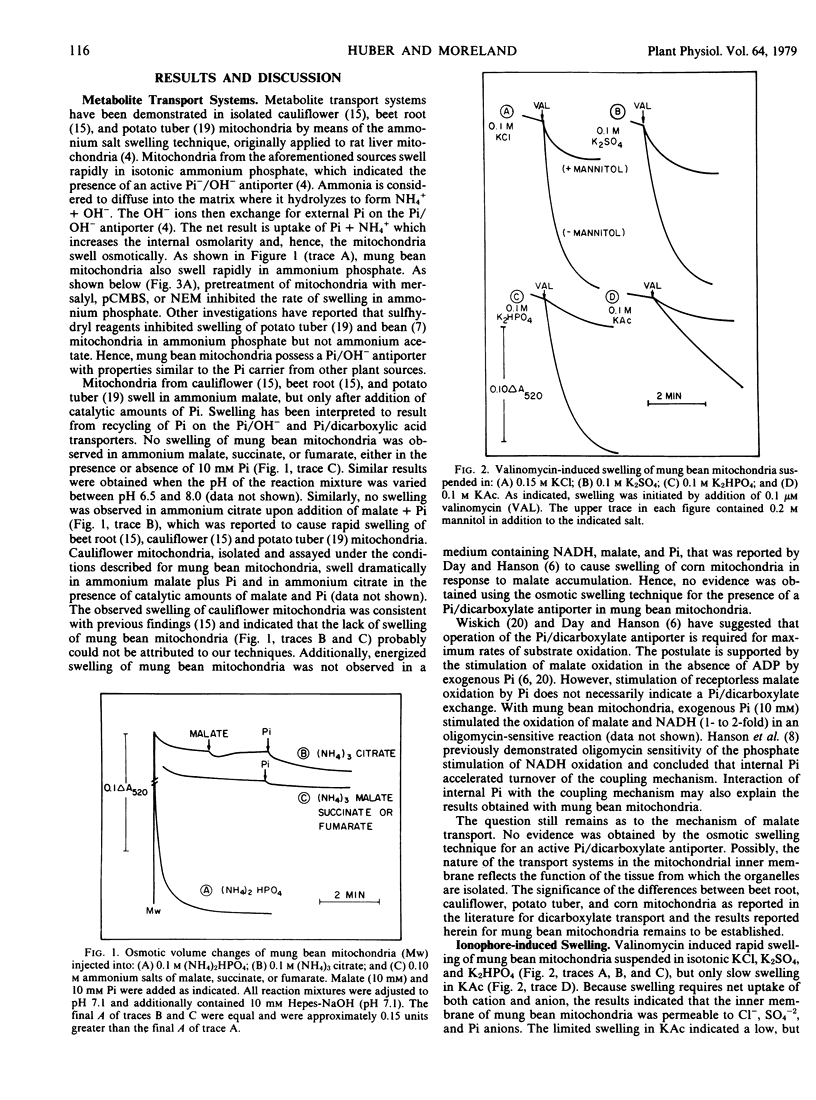
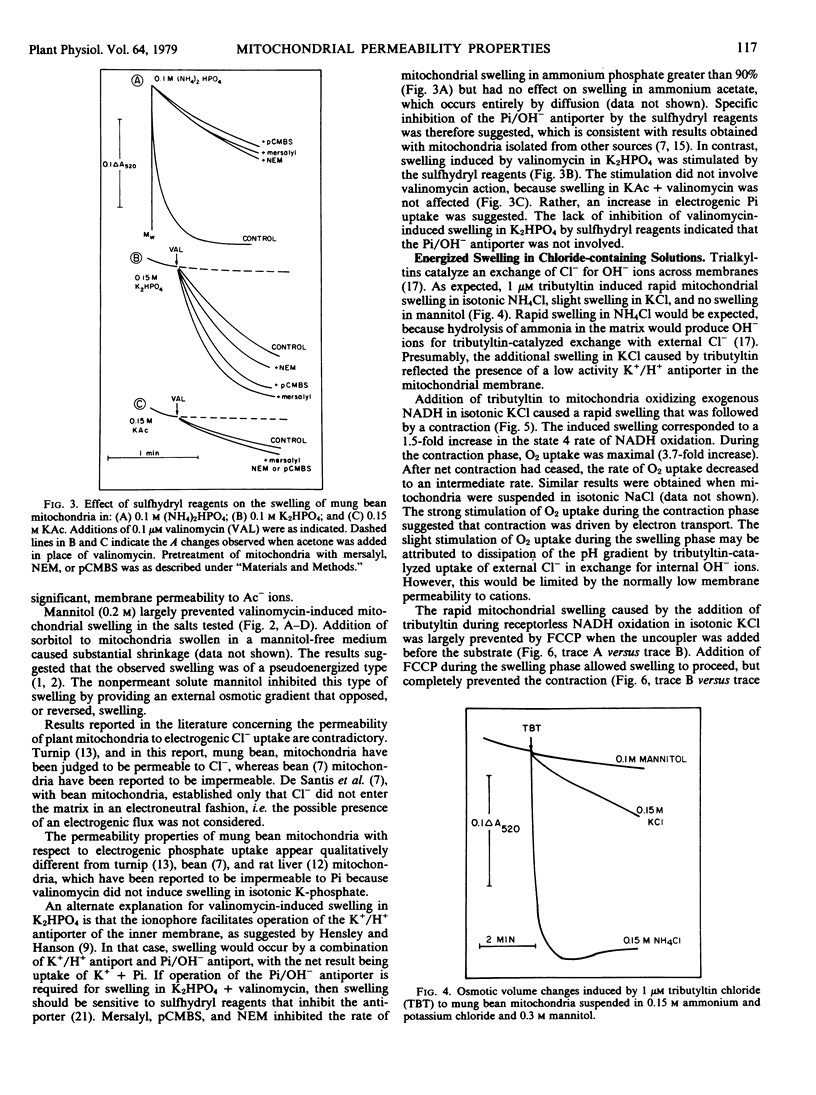
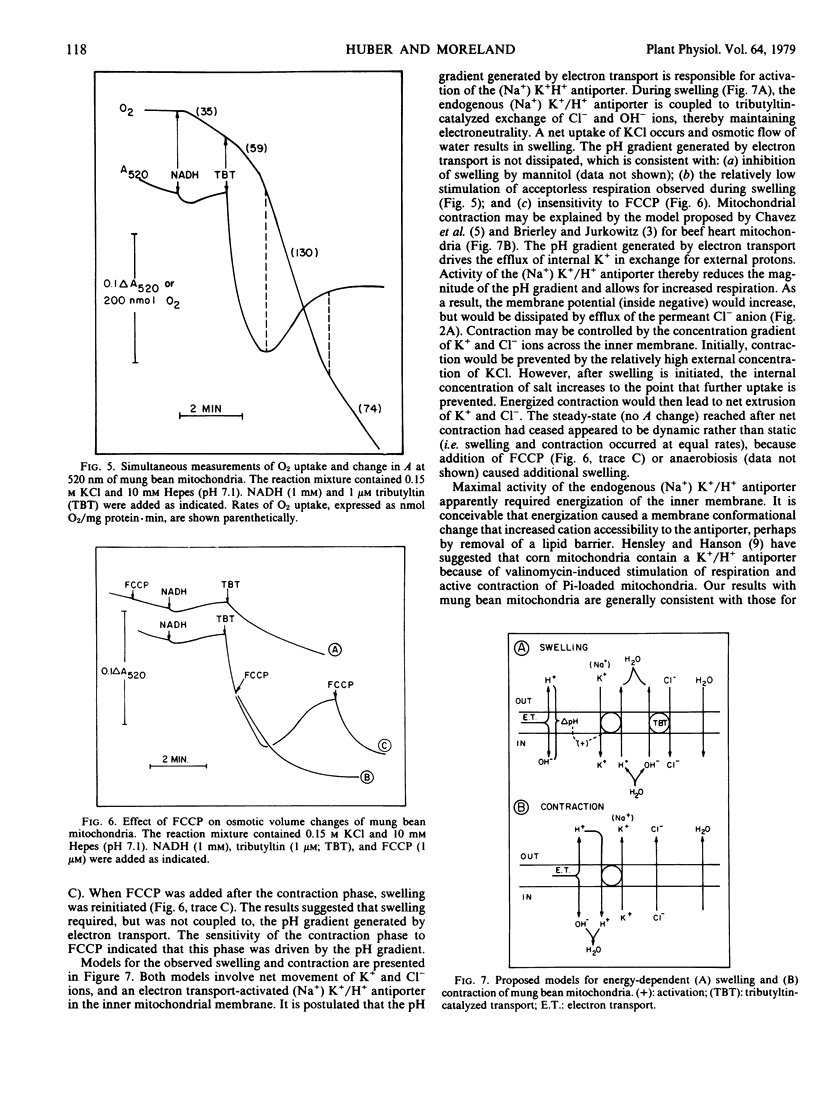
The permeability properties of the inner membrane of mung bean mitochondria were studied by osmotic swelling techniques. Rapid mitochondrial swelling was observed in isotonic ammonium phosphate, which indicated that an active phosphate/hydroxyl antiporter was present. The phosphate carrier was specifically inhibited by sulfhydryl reagents. Mitochondria did not swell in isotonic ammonium salts of malate, succinate, or fumarate, either in the presence or absence of 10 millimolar phosphate. Additionally, no swelling was observed in ammonium citrate upon addition of malate plus phosphate. Consequently, no evidence was obtained with the osmotic swelling technique for a coupled exchange of phosphate for dicarboxylic acids across the membrane.
On the basis of valinomycin-induced swelling in potassium salts, suggestions were obtained that chloride, sulfate, and phosphate anions permeated more rapidly than acetate anions. Sulfhydryl reagents increased the rate of valinomycin-induced swelling in potassium phosphate, but had no effect on swelling in potassium acetate.
Tributyltin induced a low rate of mitochondrial swelling in KCl in the absence of substrates, which indicated the presence of a low activity (Na+) K+/H+ antiporter in the membrane. In the presence of NADH, rapid swelling, followed by a contraction, was observed upon addition of tributyltin. Swelling was insensitive to uncouplers, whereas contraction was uncoupler-sensitive. O2 uptake (state 4) was greatest (3- to 4-fold stimulation) during the contraction phase, which indicated that the observed contraction was coupled to the pH gradient formed during electron transport. The results suggested that energization increased the activity of the (Na+) K+/H+ antiporter in the inner mitochondrial membrane.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asai J., Blondin G. A., Vail W. J., Green D. E. The mechanism of mitochondrial swelling. IV. Configurational changes during swelling of beef heart mitochondria. Arch Biochem Biophys. 1969 Jul;132(2):524–544. doi: 10.1016/0003-9861(69)90396-8. [DOI] [PubMed] [Google Scholar]
- Blondin G. A., Green D. E. Mechanism of mitochondrial swelling. 3. Two forms of energized swelling. Arch Biochem Biophys. 1969 Jul;132(2):509–523. doi: 10.1016/0003-9861(69)90395-6. [DOI] [PubMed] [Google Scholar]
- Chávez E., Jung D. W., Brierley G. P. Energy-dependence exchange of K+ in heart mitochondria. K+ efflux. Arch Biochem Biophys. 1977 Oct;183(2):460–470. doi: 10.1016/0003-9861(77)90381-2. [DOI] [PubMed] [Google Scholar]
- Day D. A., Hanson J. B. Effect of phosphate and uncouplers on substrate transport and oxidation by isolated corn mitochondria. Plant Physiol. 1977 Feb;59(2):139–144. doi: 10.1104/pp.59.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J. B., Bertagnolli B. L., Shepherd W. D. Phosphate-induced Stimulation of Acceptorless Respiration in Corn Mitochondria. Plant Physiol. 1972 Sep;50(3):347–354. doi: 10.1104/pp.50.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley J. R., Hanson J. B. The action of valinomycin in uncoupling corn mitochondria. Plant Physiol. 1975 Jul;56(1):13–18. doi: 10.1104/pp.56.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. I. Isolation and Some Characteristics of Tightly-coupled Mitochondria from Dark-grown Mung Bean Hypocotyls. Plant Physiol. 1967 Jan;42(1):67–75. doi: 10.1104/pp.42.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Translocation of some anions cations and acids in rat liver mitochondria. Eur J Biochem. 1969 Jun;9(2):149–155. doi: 10.1111/j.1432-1033.1969.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Moreland D. E., Boots M. R. Effects of optically active 1-(alpha-methylbenzyl)-3-(3,4-dichlorophenyl)urea on reactions of mitochondria and chloroplasts. Plant Physiol. 1971 Jan;47(1):53–58. doi: 10.1104/pp.47.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. L., Williams G. R. Anion transporters in plant mitochondria. Plant Physiol. 1973 Apr;51(4):667–670. doi: 10.1104/pp.51.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Selwyn M. J., Dawson A. P., Stockdale M., Gains N. Chloride-hydroxide exchange across mitochondrial, erythrocyte and artificial lipid membranes mediated by trialkyl- and triphenyltin compounds. Eur J Biochem. 1970 May 1;14(1):120–126. doi: 10.1111/j.1432-1033.1970.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Wilson R. H., Hanson J. B., Mollenhauer H. H. Active swelling and acetate uptake in corn mitochondria. Biochemistry. 1969 Mar;8(3):1203–1213. doi: 10.1021/bi00831a055. [DOI] [PubMed] [Google Scholar]
- Wiskich J. T. Phosphate-dependent Substrate Transport into Mitochondria: Oxidative Studies. Plant Physiol. 1975 Jul;56(1):121–125. doi: 10.1104/pp.56.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]


