Figure 3.
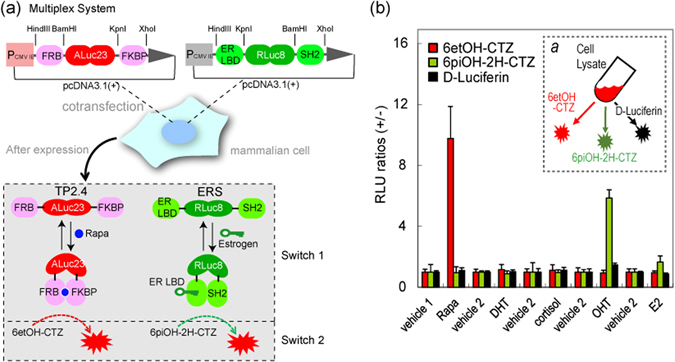
(a) Schematic diagram of a multiplex assay system with two pcDNA3.1(+) vectors encoding molecular tension probe 2.4 (TP2.4) and ER LBD-RLuc8-SH2 (ERS), respectively. After expression, TP2.4 and ERS coexist in mammalian cells, which are ready to illuminate ligand activity. The working mechanisms of TP2.4 and ERS are briefly illustrated in the box-highlighted Switches 1 and 2. Upon stimulation by a ligand, TP2.4 or ERS exerts an intramolecular protein-to-protein interaction (PPI) (Switch 1). This PPI enhances the sandwiched luciferase’s activity. The enhanced activities are pinpoint visualized with specific coelenterazine (CTZ) analogues (Switch 2). (b) Specific optical intensities of the substrates in the multiplex assay system carrying two luciferase-based probes. Conventional assays are unable to discriminate the two optical signals. However, the present multiplex system pinpoint-illuminated the rapamycin or estrogen antagonist (OHT) activities without any signal contamination. Vehicles 1 and 2 are negative controls with 0.1% alcohol and 0.1% DMSO, respectively. Inset a briefly illustrates the experimental procedure with the specific substrates. The live cells were firstly stimulated with rapamycin and estrogen, lysed, and pinpoint-illuminated with a specific luciferin. Abbreviations: FRB, the rapamycin-binding domain of mTOR; FKBP, FK506-binding protein; ER LBD, the ligand binding domain of human estrogen receptor; SH2, the SH2 domain of v-Src; Rapa, rapamycin; DHT, 5α-dihydrotestosterone; OHT, 4-hydroxytamoxifen; E2, 17β-estradiol.
