Figure 4.
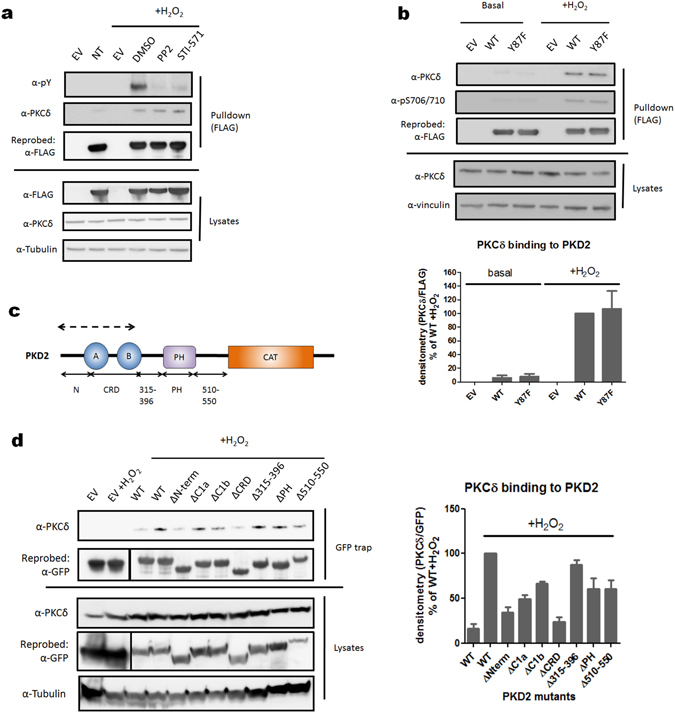
Different binding mode of PKCδ to PKD1 versus PKD2 in oxidative stress conditions. (a) Tyrosine kinase inhibition does not have an effect on the association between PKCδ and PKD2 during oxidative stress. HEK293 cells were transfected with wild type (WT) PKD2. 48 h after transfection, cells were treated with PP2 (10 µM) or STI-571 (5 µM) and subsequently stimulated with H2O2 (10 mM, 10 min) and PKD2 was precipitated from the cells using FLAG antibody. Immunoprecipitates were subjected to western blot and probed with the indicated antibodies. Western blots were cropped for clarity; uncropped images can be found in Supplementary Fig. S15. (b) Tyr-87 phosphorylation is not required for the association between PKD2 and PKCδ. HEK293 cells were transfected with wild type (WT) PKD2 or the indicated mutants. 48 h after transfection cells were stimulated with H2O2 (10 mM, 10 min) and PKD2 was precipitated from the cells using FLAG antibody. Immunoprecipitates were subjected to western blot and probed with the indicated antibodies. Quantification of three independent experiments is shown. Western blots were cropped for clarity; uncropped images can be found in Supplementary Fig. S16. (c) Schematic representation of PKD2 deletion mutants used for mapping the PKCδ binding domain. Deleted regions are denoted with full arrows. The proposed PKCδ binding region is indicated by a dotted arrow. (d) The N-terminal AP region and CRD of PKD2 are necessary for PKCδ interaction. HEK293 cells were transfected with wild type (WT) PKD2 or the indicated mutants. 48 h after transfection, cells were stimulated with H2O2 (10 mM, 10 min) and PKD2 was precipitated from the cells via GFP trap. Immunoprecipitates were subjected to western blot and probed with the indicated antibodies. Quantification of three independent experiments is shown. Western blots were cropped for clarity; uncropped images can be found in Supplementary Fig. S17.
