Figure 1.
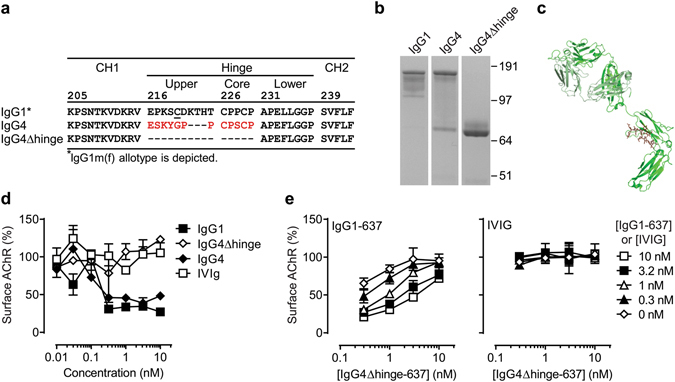
Design and in vitro characterization of IgG4Δhinge. (a) Sequence alignment of residues 205–234 (EU-numbering convention) of human IgG1, IgG4 and IgG4Δhinge. (b) Purified human IgG1, IgG4 and IgG4Δhinge variants of mAb 637 analyzed by non-reducing SDS-PAGE (image is cropped to show relevant bands, the complete gel is shown in supplementary Fig. 5). (c) In silico model of IgG4Δhinge based on crystal structures 1ADQ61 (CH2-CH3 domains), 1MCO62 (Δhinge region) and 1HZH63 (Fab region). HCΔhinge depicted in green, LC depicted in pale green. (d) AChR surface down-modulation on TE671 cells by purified variants of mAb 637 as indicated. Pooled intravenous immunoglobulin (IVIg) is included as negative control (e) Inhibition of (auto-) antibody-mediated AChR surface down-modulation on TE671 cells by IgG4Δhinge-637. Different concentrations of the challenge autoantibody (IgG1-637) or a negative control (IVIG) were tested in combination with IgG4Δhinge-637; each curve corresponds to a single concentration of challenge antibody (0–10 nM) as shown in the key on the right.
