Abstract
Autophagy is a crucial and physiological process for cell survival from yeast to mammals, including protozoan parasites. Toxoplasma gondii, an intracellular parasite, typically exploits autophagic machinery of host cell; however host cell upregulates autophagy to combat the infection. Herein we tested the efficacy of Rottlerin, a natural polyphenol with autophagic promoting properties, against Toxoplasma infection on the chorioncarcinoma-derived cell line BeWo. We found that Rottlerin, at sub-toxic doses, induced morphological and biochemical alterations associated with autophagy and decreased Toxoplasma growth in infected cells. Although autophagy was synergically promoted by Toxoplasma infection in combination with Rottlerin treatment, the use of the autophagy inhibitor chloroquine revealed that Rottlerin anti-parasitic effect was largely autophagy-independent and likely mediated by the converging inhibitory effect of Rottlerin and Toxoplasma in host protein translation, mediated by mTOR inhibition and eIF2α phosphorylation. Both events, which on one hand could explain the additive effect on autophagy induction, on the other hand led to inhibition of protein synthesis, thereby depriving Toxoplasma of metabolically essential components for multiplication. We suggest that modulation of the competition between pathogen requirement and host cell defense might be an attractive, novel therapeutic approach against Toxoplasma infection and encourage the development of Rottlerin-based new therapeutic formulations.
Introduction
Autophagy is an evolutionary conserved process of cellular degradation that is crucial for cell survival, differentiation and development from yeast to mammals, including protozoan parasites1. The autophagic process is controlled by several AuTophagy Genes (ATGs) through a multi-step mechanism that includes induction, cargo recognition, autophagosome formation/fusion with lysosomes and cargo digestion followed by release of the degradation products2. Autophagy is constitutively active on a basal level helping to sustain cellular functions, but it can be strongly induced in response to a multitude of stimuli, such as nutrients deprivation, hypoxia and pathogen infection (xenophagy), to name but a few. Although this process can be seen as a survival mechanism to deal with nutrient limitation, the prolonged and intense activation of autophagy can be lethal, because of self-degradation of essential cellular components3. Thus, autophagy can be a cell survival mechanism in certain circumstances, but a mediator of cell death in others. Autophagy is indeed a type of programmed cell death (Type II death) that has a distinct progression from that of the Type I apoptotic death and is particularly relevant in those cells where the apoptotic machinery is compromised, such as in certain cancer cells4, 5 or in protozoa, where a typical apoptosis has not been universally demonstrated6.
Toxoplasma gondii is an intracellular protozoan parasite that infects virtually any type of nucleated cells from a wide range of warm-blooded vertebrates, including humans. The prevalence of toxoplasmosis varies greatly around the world, it has been estimated that approximately 30% of human population worldwide is chronically infected with T. gondii 7. Primary infection in adults results in mild or nonspecific symptoms8. However in immunocompromised individuals, primary infection or reactivation of chronic infection can cause fatal toxoplasmicencephalitis, myocarditis and pneumonitis9. In addition, toxoplasmosis is associated with severe congenital defects when primary infection is acquired during pregnancy, especially during the first trimester of pregnancy8.
Moreover, because Toxoplasma is an obligated intracellular pathogen, the autophagic process switched on by the host cell (xenophagy) might be determinant for the parasite fate. In fact, while T. gondii typically exploits the autophagic machinery of the host cell to its own advantage10, the host cell upregulates autophagy to combat the infection. It follows that there might be a threshold, non-deadly for the host cell, beyond which autophagy leads to parasite death. Modulation of the competition between pathogen requirement and host cell defense could be therefore an attractive and novel therapeutic approach.
Following this hypothesis, in the current study, we tried to perform a precise autophagy-targeted approach, based on the known autophagy promoting effects of Rottlerin4, 10, 11.
As recently reported, Rottlerin induced autophagy through inhibition of mTORC1, a negative regulator of autophagy5. Importantly, mTORC1 is also a key player in the control of protein synthesis, which, conversely, is stimulated. Indeed, the initiation step of mRNA translation is commenced by the binding of the eukaryotic translation initiation factor 4 F (eIF4F) complex to the cap-structure of mRNA. eIF4E is regulated by 4E-BP, whose phosphorylation by mTORC1 causes the release of free eIF4E that will then initiate protein synthesis. Thus, mTORC1 inhibition results in both autophagy induction and translational arrest12.
Rottlerin is a natural polyphenol isolated from Mallotus philippinensis, an ancient traditional Indian medicinal plant in Ayurvedic Medicine System, mainly known for its anthelminthic activity13, 14. Rottlerin, however, has potential beneficial applications in a variety of other ailments, being an antioxidant15, anti-inflammatory16, 17, antiallergic17, antibacterial18, 19 and anticancer compound20. Rottlerin is a very versatile substance that has been used for decades as a selective PKCδ inhibitor21 though it is now clear that Rottlerin also inhibits other enzymes22 and acts as a mitochondrial uncoupler23.
Given the pleiotropic properties of Rottlerin, in particular its autophagy inducing ability and the importance of T. gondii infection during pregnancy8, the aim of the current study was to test the efficacy of Rottlerin against the parasite, in the trophoblast-like cell line BeWo, the most extensively used cellular in vitro model for villous trophoblast studies.
Autophagy is physiologically involved in normal placentation24 and recent studies suggest that autophagy in trophoblasts has primarily an adaptive role25. An increase in autophagy flux is indeed associated with cytoprotective mechanisms of the trophoblast cells against micro environmental challenges24, 26. Hence, autophagy induction is expected to be well tolerated by BeWo cells.
However, because of the several, potentially harmful, Rottlerin effects, the drug needs to be used with caution. Then, the main challenge of this work was to individuate the optimal dose that is not toxic for the host cell, but is lethal for the parasite.
Results
BeWo cells viability
The effect of Rottlerin on viability of BeWo cells was evaluated by Trypan Blue exclusion assay. As reported in Fig. 1, no difference in cell viability with respect to control (vehicle-treated cultures), was observed when BeWo cells were treated with Rottlerin at concentrations from 0.5 to 5 µM, neither at 24 nor at 48 hours. On the other hand, significant decrease of cell viability was observed at the concentrations of 10 and 20 µM at both 24 and 48 hours (Fig. 1). Specifically, cell viability was reduced to around 60% after 24 hours of 10 µM Rottlerin treatments and to less than 50% after 48 hours. The decrease was more marked with 20 µM Rottlerin, being cell viability reduced to less than 50% already after 24 hours of incubation, and to around 30% after 48 hours. Based on these data we performed subsequent experiments using Rottlerin at concentrations of 0.5, 1, 2 and 5 µM.
Figure 1.
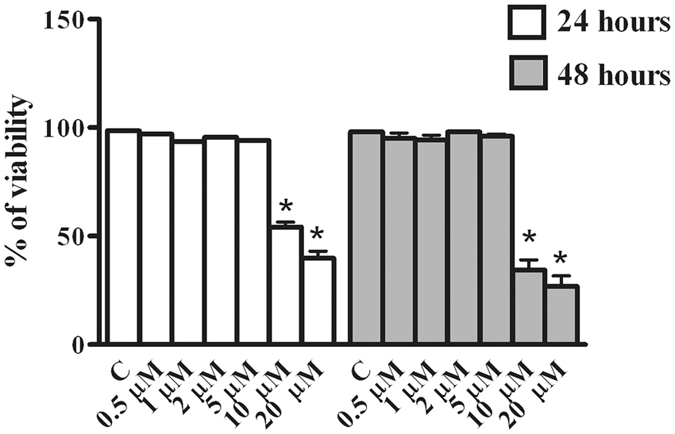
Effect of Rottlerin treatment on BeWo cells viability. BeWo cells were treated for 24 and 48 hours with increasing concentrations of Rottlerin (0.5 µM–20 µM) and cell viability was evaluated by direct cell counting (Trypan Blue). Data are expressed as a percentage of control (vehicle-treated)cultures and reported as mean ± SEM. The results represent four independent experiments performed in quadruplicate for each data point. *p < 0.05.
Rottlerin decreases T. gondii growth in BeWo cells
The effect of Rottlerin on parasite growth was evaluated in BeWo cells infected for 3 hours and then treated with the compound for 24 and 48 hours. Our results showed that increasing concentrations of Rottlerin resulted in significant inhibition of tachyzoites growth from around 50% (0.5 µM Rottlerin) to 85% (5 µM Rottlerin) as respect to vehicle-treated cell cultures after 24 as well as after 48 hours cultures (Fig. 2). Interestingly, while a significant increase was observed in tachyzoites number from 24 to 48 hours in 0.5, 1 and 2 µM Rottlerin-treated cultures, no evidence of parasite growth was found in the same time interval at the dose of 5 µM (Fig. 2). Therefore, this dose was chosen for the subsequent experiments. The indices of infection and parasite replication were determined in BeWo after 24 h of infection with or without Rottlerin treatment by a direct observation under a light microscope (see Supplementary Figure S1). Results showed a significant decrease of infected cells as well as a significant decrease in the number of parasites per cells (Supplementary Figure S1).
Figure 2.
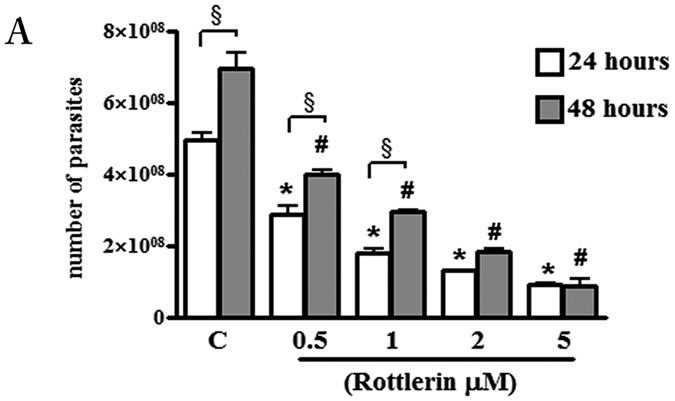
Effect of Rottlerin treatment on T. gondii proliferation. BeWo cells were infected with T. gondii (host cell: tachyzoites, 1:5) for 3 hours then treated with increasing concentrations of Rottlerin (0.5 µM–5 µM). T. gondii proliferation was evaluated after 24 and 48 hours by a colorimetric microtiter assay using β-galactosidase-expressing tachyzoites. Data were expressed as mean ± SEM of the number tachyzoites calculated in relation to a reference curve and are representative of three independent experiments performed in triplicate. *p < 0.0001: Rottlerin -treated vs control-vehicle cultures at 24 hours; #p < 0.01: Rottlerin -treated vs control-vehicle cultures at 48 hours; §p < 0.05: Rottlerin-treated cultures at 24 hours vs 48 hours.
Toxoplasma gondii and Rottlerin are able to induce morphological and biochemical alterations associated with autophagy in BeWo Cells
Due to the well-known properties of Rottlerin as a pro-autophagic drug, we performed morphological and biochemical analysis to investigate on the significant reduction of parasites number observed in the current study.
Electron microscopy (Fig. 3 left panel) reveled that BeWo cells untreated and not infected showed typical morphological characteristics with nuclear decondensed chromatin and evident nucleolus, cytoplasm containing mitochondria, polyribosomes and granular endoplasmic reticulum (Fig. 3A–C). When BeWo cells were treated with 5 µM Rottlerin for 24 hours, morphological changes were observed with the occurrence of cytoplasmic vacuoles and multivesicular bodies (Fig. 3D–F) but no signs of nuclear alterations were visible (Fig. 3D). BeWo cells infected with T. gondii showed a slight expansion of the rough endoplasmic reticulum (Fig. 3G–I). Of note, when BeWo cells were infected with T. gondii and treated with 5 µM Rottlerin for 24 hours, dramatic morphological alterations, with many cytoplasmic vacuoles as well as numerous multivesicular bodies, were observed (Fig. 3L,M). Of note, the presence of double membraned vacuoles indicates their autophagosomal nature (Fig. 3N and O), which was confirmed by the immunoblotting analysis of cell lysates showing an increase in the lipidated form of LC3 (LC3II) and a reduction/degradation of p62 protein, hallmarks of autophagic flux, both in Rottlerin treated and infected BeWo cells (Fig. 3P and Q). Importantly, as it can be appreciated in Fig. 3Q, the expression of p62 was more markedly reduced and LC3II enhanced when BeWo cells were T. gondii infected and then treated with 5 µM Rottlerin.
Figure 3.
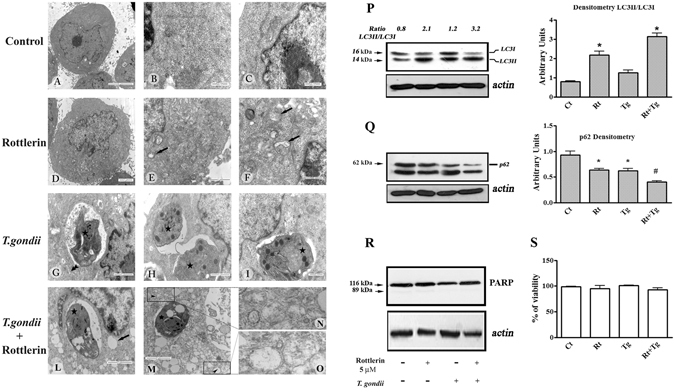
T. gondii and Rottlerin induce morphological and biochemical alterations associated with autophagy in BeWo Cells. Left panel: control-vehicle treated cultures (A,B and C); 5 µM Rottlerin-treated cultures (D,E and F); T. gondii infected cultures (G,H and I); T. gondii infected and 5 µM Rottlerin treated cultured (L,M,N and O). Arrows: cytoplasmic vacuoles; star: T. gondii; double arrow head: expansion of the rough endoplasmic reticulum; arrow head: double membraned vacuoles (P and Q): representative western blot and densitometric analysis performed on three independent experiments of LC3I/II (P) and p62 (Q) proteins in BeWo cells after infection and treatment with 5 µM Rottlerin. *p < 0.05; #p < 0.01. (R): representative western blot of three independent experiments of PARP proteins in BeWo cells after infection and treatment with 5 µM Rottlerin. (S): viability of BeWo cells after infection and treatment with 5 µM Rottlerin (Rt). (A) Bar = 5 µm; (B,C,F and I), Bar = 500 nm; (D,L and M), Bar = 2 µm; (E,G,H), Bar = 1 µm.
At the same time, we found that BeWo cell viability was unaffected by the infection and 5 µM Rottlerin treatment or a combination of the two (Fig. 3S). This result, along with the lack of nuclear alterations (Fig. 3D) and PARP cleavage (Fig. 3R), excludes the occurrence of apoptotic cell death at this Rottlerin dose.
Beclin-1 and mTORC1 involvement in Rottlerin- and Toxoplasma-triggered autophagy
The two main pathways eventually involved in the autophagy process, Beclin-1, the mammalian orthologue of yeast Atg6/Vps30 with a key role in autophagosome formation27 and the mTORC1 cascade28 were investigated. As shown in Fig. 4A, no change (induction) of Beclin-1 was observed both in the Rottlerin-treated and in the T. gondii -infected cells. Conversely, Rottlerin treatment, but not T. gondii infection, decreased the levels of the phosphorylated forms of 4EBP-1 isoforms (Fig. 4B), indicating that, in agreement with previous findings5, Rottlerin induced autophagy via inhibition of mTORC1 kinase activity. Given the independence from both Beclin-1 and mTORC1 in T. gondii-driven autophagy in the host cell, we presumed the involvement of the endoplasmic reticulum (ER) stress/eIF2α axis as a likely mechanism29. The results from Western blotting analysis of BeWo cell lysates after infection, indicated the ER stress-stimulated kinase, PERK, was activated, since eIF2α phosphorylation (a marker of ER stress) increased (Fig. 4C). Moreover, in agreement with the literature30, we found that Rottlerin alone was able to trigger ER stress and to increase eIF2α phosphorylation.
Figure 4.
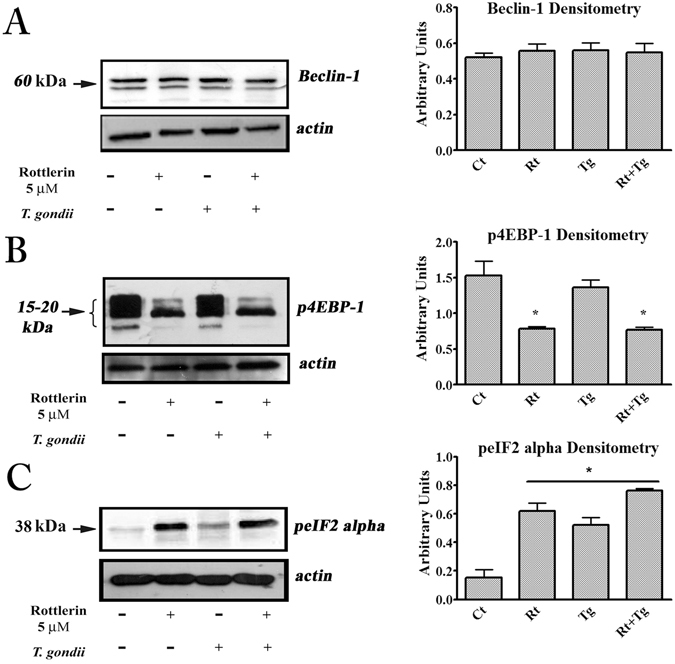
T. gondii and Rottlerin engage different autophagic pathways. Representative western blot and densitometric analysis, performed on three independent experiments, of Beclin-1 (A), phosphorylated forms of 4EBP-1 (B) and phosphorylated eIF2α (C) in BeWo cells after infection and treatment with 5 µM Rottlerin. *p < 0.05.
Role of host autophagy in T. gondii proliferative potential
To evaluate the role of autophagy in the Rottlerin anti-parasitic action, the autophagy inhibitor CQ, which blocks the late acidification steps of the process, was used. As shown in Fig. 5, CQ failed to prevent the Rottlerin inhibition of parasite replication, indicating that, although the autophagic pathway was clearly stimulated, autophagy was not responsible for the Rottlerin protective mechanism against infection. Moreover, in agreement with the notion that T. gondii usually exploits the autophagic machinery of the host cell for survival purposes, CQ alone decreased parasite growth inside the host cell.
Figure 5.
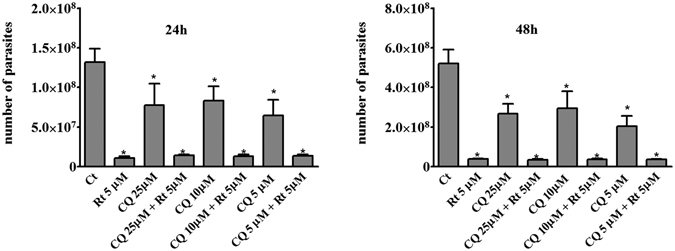
Autophagy is not responsible for the Rottlerin anti parasitic action. BeWo cells were infected with T. gondii (host cell: tachyzoites, 1:5) for 3 hours then treated 5 µM Rottlerin plus increasing concentrations of CQ (5, 10, 25 µM). T. gondii proliferation was evaluated as described in Fig. 2 after 24 and 48 hours *p < 0.0001. Data were expressed as mean ± SEM of the number of tachyzoites calculated in relation to a reference curve and are representative of three independent experiments performed in quintuplicate.
Discussion
Up to now, an effective treatment against T. gondii, with no toxicity for the host, has not been yet discovered. In fact, most of the current therapies exhibit a high degree of toxicity8, 31.
The recommended treatment adopted for toxoplasmosis is the combination of the antibiotics sulfadiazine, pyrimethamine and folinic acid or spiramycin. However, this regimen is commonly associated with many adverse effects, including teratogenic effects in the first trimester of pregnancy32. Thus, researchers are encouraged to disclose new, less toxic drugs that actively combat/prevent T. gondii infection.
In the current study, BeWo cells were used as an in vitro model of human trophoblast. Our group for many years is engaged in the study of congenital toxoplasmosis focusing the research on the placenta33, 34. Many in vitro models have been established for human placental trophoblast studies, among which cell lines, including BeWo cells, are widely used and particularly advantageous in terms of reproducibility to evaluate the net effect of therapeutic agents on laboratory challenge tests with pathogens35, 36. We found that Rottlerin sub-toxic concentrations for host cells are able to combat T. gondii infection in BeWo cells significantly and dose dependently.
In agreement with the notion that Rottlerin is an autophagy inducer, we have shown that Rottlerin alone, even at doses not affecting cell viability, can induce autophagy also in BeWo cells. As previously found in MCF-7 cells, also in BeWo cells Rottlerin induced autophagy via mTORC1 inhibition and independently from Beclin-15. Autophagy is a natural defense against parasites and, indeed, we found enhanced cytoplasmic vacuolization and stimulated autophagic flux in infected cells. However, T. gondii -induced autophagy was both Beclin-1 and mTORC1-independent, indicating that the two autophagy inducers engage different pathways. Recent studies by Sellek et al.37 reported that the IFNγ-mediated T. gondii control in human cells occurred through a mechanism different from both canonical autophagy (Beclin 1-independent) and xenophagy. Despite the different experimental model, Sellek’s and our results converge on a non-canonical (Beclin 1-independent) autophagic-like pathway triggered by T. gondii in host cells.
Our results are also in agreement with the earlier Wang’s study38 which demonstrated that T. gondii-induced autophagy in host cells was mTOR-independent and somehow dependent on a calcium-sensitive step.
Although further work is needed to identify the exact mechanism of T. gondii-induced host autophagy, the slight expansion of the endoplasmic reticulum (ER) observed in infected BeWo cells (Fig. 3G–I), pointed towards a possible T. gondii-induced ER stress/eIF2 kinase (PERK)-dependent autophagic pathway. Indeed, eIF2 kinases can promote autophagy, via phospho-eIF2α-ATF4, by inducing transcription of a set of autophagy genes29.
The association between the parasitophorous vacuoles (PV) and the host ER, which could be a source of membrane for the enlargement of the PV, dates back to 199739 and it has been confirmed in more recent studies showing, by electron microscopy, that PVs were surrounded by ER40. It is very possible that this tight interaction could led to ER stress and trigger the so called unfolding protein response in the host. Consistently, recent studies reported that apoptosis of mice trophoblasts and neural stem cells infected with T. gondii was triggered by ER stress41, 42. Although no (apoptotic) death was observed in infected BeWo cells, the results from Western blotting analysis of cell lysates after infection showed increased eIF2α phosphorylation, indicating the activation of the ER stress-stimulated kinase, PERK. We assumed that this could be the underlying mechanism of T. gondii-triggered host autophagy.
Accordingly to the double stimulation (by Rottlerin and infection-induced ER stress and Rottlerin-inhibited mTORC1), we observed an exacerbated autophagic response, both at the microscopic (TEM) and the biochemical (Western blotting of LC3I/II and p62) level, in infected cells after Rottlerin exposure.
However, the over-stimulation of autophagy was not the mechanism by which Rottlerin decreased parasite replication, since the drug had superimposable effects in the absence or in the presence of the autophagy inhibitor CQ. Moreover, consistently with the notion that T. gondii usually exploits the autophagic machinery of the host cell for survival purposes, we observed that CQ alone hampered parasite growth inside the host cell. However, given the general ability of CQ to inhibit organelles acidification, we cannot exclude that it could have a direct effect on parasite as well. Moreover, because CQ does not block the formation of autophagosoma, it is possible, in theory, that this could be sufficient to arrest parasite replication even in the absence of a complete autophagic flux. However, because Rottlerin and CQ have not an additive effect, this eventuality can be ruled out. At the same time, the lack of synergism/antagonism between Rottlerin and CQ confirms the irrelevance of autophagy induction in the Rottlerin outcome.
Overall, these findings suggest that a novel mechanism, alternative to autophagy, are responsible for the Rottlerin protective effect against T. gondii infection in BeWo cells; such a mechanism could be host protein synthesis curtailing (Fig. 6).
Figure 6.
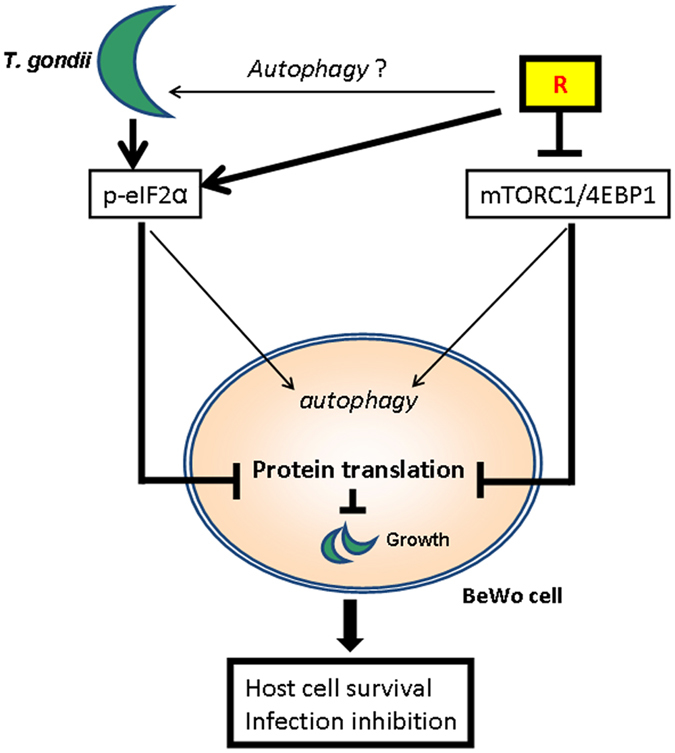
Schematic representation of the Rottlerin action against Toxoplasma gondii infection. Rottlerin inhibited the mTORC1/4EBP-1 axis and, synergically with T. gondii, induced eIF2α phosphorylation. Both events led to autophagy induction and inhibition of protein synthesis.
Indeed, in addition to mTORC1, which is a regulator of cap-dependent mRNA translation, phosphorylation of eukaryote initiation factor 2a (eIF2a) on serine 51 by ER stress-activated kinases potently inhibits the initiation of protein translation. It is well known that T. gondii actively appropriates numerous host cell processes and significant changes in host cell transcripts occur during the course of the infection, indicating that the contribution of host transcriptional/translational activities is essential for successful infection43.
The inhibition of protein translation, that inevitably occurs in concomitance with autophagy induction, appeared to be a very effective process in reducing parasites proliferation, especially when synergically promoted by T. gondii infection, via ER stress/eIF2α, in combination with Rottlerin, via mTORC1/4EBP.
In closing, the results of the current study encourage the development of Rottlerin-based new therapeutic formulations against T. gondii infection for which a limited number of effective and safe drugs are currently available. Particularly, the results here obtained might be useful to combat and/or prevent the possible severe consequences for the fetus by maternal infection during pregnancy.
Materials and Methods
Cell culture
The choriocarcinoma-derived BeWo cell line, used as experimental model of trophoblast cells, was obtained from the American Type Culture Collection, (Manassas, VA, USA). Cells were grown in RPMI 1640 medium (Cultilab, Campinas, SP, Brazil) containing 10% fetal bovine serum (FBS) (Cultilab) supplemented with 25 mM HEPES, 2 mM L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin (Sigma Chemical Co., St Louis, MO, USA). Cells were routinely cultured in 75 cm2 vented flasks in a humidified 5% CO2–95% air atmosphere at 37 °C until 70–80% confluence.
Rottlerin
Rottlerin (Sigma Chemical Co.) was dissolved in dimethyl sulfoxide (DMSO; vehicle) to a concentration of 20 mM (stock solution). Stock solution was freshly diluted in culture medium and concentrations ranging from 0.5–20 µM were used for the treatments.
Cell viability
Cell viability was assessed by Trypan blue exclusion assay. Briefly, BeWo cells were seeded in 24-well plates at 5 × 104/mL in RPMI 1640 (supplemented as above described) and 10% FBS. The day after, the medium was substituted with fresh RPMI 1640 with 2% of FBS containing increasing concentrations of Rottlerin (0.5, 1, 2, 5, 10 and 20 µM) or vehicle as control. The cultures were incubated for 24 and 48 hours. Cell viability was assessed also after cell infection. Briefly, BeWo cells were infected and treated with Rottlerin (5 µM) for 24 hours as described thereafter. The number of viable cells was evaluated by detaching with trypsin solution (0.05% trypsin–0.02% sodium EDTA) and using the Countess™ automated cell counter (Invitrogen, Carlsbad, CA, USA), which employs the standard Trypan blue technique. Four replicate counts were determined at each time point. The viability was presented as a percentage of control (vehicle-treated cells).
Parasite
Tachyzoites of T. gondii 2F1 strain derived from the RH strain and gently provided by Dr. Vern Carruthers, Medical School of Michigan University (USA) were used in the current study. These parasites, which constitutively express cytoplasmic β-galactosidase, were propagated in BeWo cells maintained in RPMI 1640 medium supplemented with penicillin, streptomycin, and 2% FBS at 37 °C and 5% CO2.
Tachyzoites were harvested by scraping off the BeWo monolayer within 3 days of infection, passed through a 26-gauge needle to lyse any remaining intact host cells and finally centrifuged at 720 × g for 5 minutes to pellet the parasite. Parasites, suspended in RPMI 1640 medium, were stained with 0.4% Trypan blue and counted in a hemocytometric chamber for infection experiments.
Infection of BeWo cells by T. gondii and treatments
BeWo cells were cultured in 96-well plates (2 × 104 cells/well/200 µL) in RPMI 1640 with 10% FBS at 37 °C and 5% CO2 overnight. Next, BeWo cells were infected with T. gondii tachyzoites (2F1 strain) at levels of 5 parasites per cell (5:1). After a 3 hour-infection period, the non-adherent parasites were removed along with the supernatant. The infected cells were washed twice with warm medium, the plates were refilled with fresh medium at 2% of FBS containing the treatment (Rottlerin at 0.5, 1, 2 and 5 µM), and incubated for 24 and 48 hours at 37 °C. In a set of experiments, the infected cells were treated with 5 µM Rottlerin plus 5, 10 and 25 µM chloroquine (CQ) (Sigma Aldrich, St. Louis, MO USA).
T. gondii intracellular proliferation was evaluated using a colorimetric β-galactosidase assay as previously described44. T. gondii intracellular proliferation data were expressed as the number of tachyzoites calculated in relation to the standard curve of 2F1 strain tachyzoites ranging from 1 × 106 to 15.625 × 103 parasites.
Electron Microscopy
BeWo cells, under the various treatments, were pelleted and fixed overnight with 2.5% glutaraldehyde, 2% paraformaldehyde diluted in 0.1 M phosphate buffered saline (PBS), pH 7.2, washed three times with PBS and post-fixed for 1 hour in 1% OsO4, dehydrated in increasing concentrations of ethanol and embedded in Epon (Sigma and Co.). Ultrathin sections were stained with uranyl acetate and lead citrate and finally examined in a Zeiss EM 109 transmission electron microscope (Zeiss, Jena, Germany).
Western blotting
After treatments, BeWo were washed with PBS and then cryolysed (three freeze-thaw cycles) with RIPA [50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 1% (w/v) sodium deoxycholate, and 0.1% (w/v) sodium dodecyl sulfate (SDS), pH 7.5] containing a cocktail of protease and phosphatase inhibitors. Cell debris was pelleted by centrifugation at 14.000 × g for 15 min. The supernatants were transferred to new tubes, and the protein concentration was measured using the Bradford assay.
Equal amounts of protein samples were resolved on 10%, SDS–polyacrylamide gels under reducing condition. Proteins were electro-transferred onto PVDF membranes and then blocked in 4% non-fat dry milk in Tris-buffered saline pH 7.2 (TBS) containing 0.1% Tween 20 for 2 hours at room temperature. Then, the blots were probed with polyclonal antibodies anti human LC3I/II, p4EBP-1, Beclin 1, peIF2α and PARP (Cell Signaling Technology, Boston, MA USA), SQSTM1/p62 and β-actin (Santa Cruz Biotechnology; Santa Cruz, CA) at dilution of 1:1000 overnight at 4 °C. After washing, appropriate horseradish peroxidase-conjugated IgG was added for 2 hours at room temperature. The blots were then developed by the ECL Detection Reagents and then exposed on photographic film, according to the manufacturer’s instructions or digitalized with CHEMI DOC Quantity One program (BioRad Microscience).
Statistical analysis
All experiments were repeated at least three times. The data were analyzed with GraphPad Prism 4 using one-way ANOVA and the Bonferroni multiple comparison post-test or Student’s t-test when appropriate. p < 0.05 was considered to be statistically significant.
Electronic supplementary material
Infection of BeWo cells by T. gondii and treatments
Acknowledgements
This work was supported by the following Brazilian Research Agencies: CNPq, FAPEMIG, and CAPES.
Author Contributions
F.I., E.M. and E.A.V.F. were responsible for conceiving, designing and performing the experiments, as well as interpreting the results and writing the manuscript; E.D., J.G.O., R.J.S., R.R., and L.C. performed experiments; A.M.A. was responsible for data acquisition; L.P. and J.R.M. intellectually contributed to the study; B.F.B. and A.O.G. assisted in designing experiments and interpreting results.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Francesca Ietta and Emanuela Maioli contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-01525-6
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kiel JA. Autophagy in unicellular eukaryotes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010;365:819–30. doi: 10.1098/rstb.2009.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pyo JO, Nah J, Jung YK. Molecules and their functions in autophagy. Exp. Mol. Med. 2012;44:73–80. doi: 10.3858/emm.2012.44.2.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilkinson S, Ryan KM. Autophagy: an adaptable modifier of tumourigenesis. Curr. Opin. Genet. Dev. 2010;20:57–64. doi: 10.1016/j.gde.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Torricelli C, et al. Alternative Pathways of Cancer Cell Death by Rottlerin: Apoptosis versus Autophagy. Evid. Based Complement Alternat. Med. 2012;2012:980658–11. doi: 10.1155/2012/980658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torricelli C, et al. Phosphorylation-independent mTORC1 inhibition by the autophagy inducer Rottlerin. Cancer Lett. 2015;360:17–27. doi: 10.1016/j.canlet.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 6.Luder CG, Campos-Salinas J, Gonzalez-Rey E, van Zandbergen G. Impact of protozoan cell death on parasite-host interactions and pathogenesis. Parasit. Vectors. 2010;3:116. doi: 10.1186/1756-3305-3-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moncada PA, Montoya JG. Toxoplasmosis in the fetus and newborn: an update on prevalence, diagnosis and treatment. Expert Rev. Anti Infect. Ther. 2012;10:815–28. doi: 10.1586/eri.12.58. [DOI] [PubMed] [Google Scholar]
- 8.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–76. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 9.Saadatnia G, Golkar M. A review on human toxoplasmosis. Scand. J. Infect. Dis. 2012;44:805–14. doi: 10.3109/00365548.2012.693197. [DOI] [PubMed] [Google Scholar]
- 10.Gao D, et al. Autophagy activated by Toxoplasma gondii infection in turn facilitates Toxoplasma gondii proliferation. Parasitol. Res. 2014;113:2053–8. doi: 10.1007/s00436-014-3853-5. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh D, Walton JL, Roepe PD, Sinai AP. Autophagy is a cell death mechanism in Toxoplasma gondii. Cell. Microbiol. 2012;14:589–607. doi: 10.1111/j.1462-5822.2011.01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar V, et al. Functional interaction between RAFT1/FRAP/mTOR and protein kinase cdelta in the regulation of cap-dependent initiation of translation. EMBO J. 2000;19:1087–97. doi: 10.1093/emboj/19.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta SS, Verma P, Hishikar K. Purgative and anthelmintic effects of Mallotus philippinensis in rats against tape worm. Indian J. Physiol. Pharmacol. 1984;28:63–6. [PubMed] [Google Scholar]
- 14.Srivastava MC, Singh SW, Tewari JP. Anthelmintic activity of Mallotus philippinensis-kambila powder. Indian J. Med. Res. 1967;55:746–8. [PubMed] [Google Scholar]
- 15.Maioli E, et al. Rottlerin inhibits ROS formation and prevents NFkappaB activation in MCF-7 and HT-29 cells. J. Biomed. Biotechnol. 2009;2009:742936–7. doi: 10.1155/2009/742936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishii R, Horie M, Saito K, Arisawa M, Kitanaka S. Inhibitory effects of phloroglucinol derivatives from Mallotus japonicus on nitric oxide production by a murine macrophage-like cell line, RAW 264.7, activated by lipopolysaccharide and interferon-gamma. Biochim. Biophys. Acta. 2001;1568:74–82. doi: 10.1016/S0304-4165(01)00203-3. [DOI] [PubMed] [Google Scholar]
- 17.Ishii R, Horie M, Saito K, Arisawa M, Kitanaka S. Prostaglandin E(2) production and induction of prostaglandin endoperoxide synthase-2 is inhibited in a murine macrophage-like cell line, RAW 264.7, by Mallotus japonicus phloroglucinol derivatives. Biochim. Biophys. Acta. 2002;1571:115–23. doi: 10.1016/S0304-4165(02)00200-3. [DOI] [PubMed] [Google Scholar]
- 18.Zaidi SF, et al. Potent bactericidal constituents from Mallotus philippinensis against clarithromycin and metronidazole resistant strains of Japanese and Pakistani Helicobacter pylori. Biol. Pharm. Bull. 2009;32:631–6. doi: 10.1248/bpb.32.631. [DOI] [PubMed] [Google Scholar]
- 19.Shivshankar P, Lei L, Wang J, Zhong G. Rottlerin inhibits chlamydial intracellular growth and blocks chlamydial acquisition of sphingolipids from host cells. Appl. Environ. Microbiol. 2008;74:1243–9. doi: 10.1128/AEM.02151-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maioli E, Torricelli C, Valacchi G. Rottlerin and cancer: novel evidence and mechanisms. ScientificWorldJournal. 2012;2012:350826–11. doi: 10.1100/2012/350826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gschwendt M, et al. Rottlerin, a novel protein kinase inhibitor. Biochem. Biophys. Res. Commun. 1994;199:93–8. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- 22.Bain J, et al. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soltoff SP. Rottlerin is a mitochondrial uncoupler that decreases cellular ATP levels and indirectly blocks protein kinase Cdelta tyrosine phosphorylation. J. Biol. Chem. 2001;276:37986–92. doi: 10.1074/jbc.M105073200. [DOI] [PubMed] [Google Scholar]
- 24.Hung TH, Hsieh TT, Chen SF, Li MJ, Yeh YL. Autophagy in the human placenta throughout gestation. PLoS One. 2013;8:e83475. doi: 10.1371/journal.pone.0083475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanninen TT, de Andrade Ramos BR, Witkin SS. The role of autophagy in reproduction from gametogenesis to parturition. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013;171:3–8. doi: 10.1016/j.ejogrb.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 26.Delorme-Axford E, Bayer A, Sadovsky Y, Coyne CB. Autophagy as a mechanism of antiviral defense at the maternal-fetal interface. Autophagy. 2013;9:2173–4. doi: 10.4161/auto.26558. [DOI] [PubMed] [Google Scholar]
- 27.Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2:330–5. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pattingre S, Espert L, Biard-Piechaczyk M, Codogno P. Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie. 2008;90:313–23. doi: 10.1016/j.biochi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 29.B’Chir W, et al. The eIF2alpha/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013;41:7683–99. doi: 10.1093/nar/gkt563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim JH, et al. Rottlerin induces pro-apoptotic endoplasmic reticulum stress through the protein kinase C-delta-independent pathway in human colon cancer cells. Apoptosis. 2008;13:1378–85. doi: 10.1007/s10495-008-0264-z. [DOI] [PubMed] [Google Scholar]
- 31.Martins-Duarte ES, Urbina JA, de Souza W, Vommaro RC. Antiproliferative activities of two novel quinuclidine inhibitors against Toxoplasma gondii tachyzoites in vitro. J. Antimicrob. Chemother. 2006;58:59–65. doi: 10.1093/jac/dkl180. [DOI] [PubMed] [Google Scholar]
- 32.Montoya JG, Remington JS. Management of Toxoplasma gondii infection during pregnancy. Clin. Infect. Dis. 2008;47:554–66. doi: 10.1086/590149. [DOI] [PubMed] [Google Scholar]
- 33.Barbosa BF, et al. Susceptibility to Toxoplasma gondii proliferation in BeWo human trophoblast cells is dose-dependent of macrophage migration inhibitory factor (MIF), via ERK1/2 phosphorylation and prostaglandin E2 production. Placenta. 2014;35:152–62. doi: 10.1016/j.placenta.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 34.Ferro EA, et al. Macrophage migration inhibitory factor is up-regulated in human first-trimester placenta stimulated by soluble antigen of Toxoplasma gondii, resulting in increased monocyte adhesion on villous explants. Am. J. Pathol. 2008;172:50–8. doi: 10.2353/ajpath.2008.070432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franco PS, et al. Azithromycin and spiramycin induce anti-inflammatory response in human trophoblastic (BeWo) cells infected by Toxoplasma gondii but are able to control infection. Placenta. 2011;32:838–44. doi: 10.1016/j.placenta.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Ayouba A, et al. Specific stimulation of HIV-1 replication in human placental trophoblasts by an antigen of Plasmodium falciparum. AIDS. 2008;22:785–7. doi: 10.1097/QAD.0b013e3282f560ee. [DOI] [PubMed] [Google Scholar]
- 37.Selleck EM, et al. A Noncanonical Autophagy Pathway Restricts Toxoplasma gondii Growth in a Strain-Specific Manner in IFN-gamma-Activated Human Cells. MBio. 2015;6:e01157–15. doi: 10.1128/mBio.01157-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Weiss LM, Orlofsky A. Host cell autophagy is induced by Toxoplasma gondii and contributes to parasite growth. J. Biol. Chem. 2009;284:1694–701. doi: 10.1074/jbc.M807890200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinai AP, Webster P, Joiner KA. Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: a high affinity interaction. J. Cell Sci. 1997;110(Pt 17):2117–28. doi: 10.1242/jcs.110.17.2117. [DOI] [PubMed] [Google Scholar]
- 40.Magno RC, Straker LC, de Souza W, Attias M. Interrelations between the parasitophorous vacuole of Toxoplasma gondii and host cell organelles. Microsc. Microanal. 2005;11:166–74. doi: 10.1017/S1431927605050129. [DOI] [PubMed] [Google Scholar]
- 41.Wang T, et al. Toxoplasma gondii induce apoptosis of neural stem cells via endoplasmic reticulum stress pathway. Parasitology. 2014;141:988–95. doi: 10.1017/S0031182014000183. [DOI] [PubMed] [Google Scholar]
- 42.Xu X, et al. Reactive oxygen species-triggered trophoblast apoptosis is initiated by endoplasmic reticulum stress via activation of caspase-12, CHOP, and the JNK pathway in Toxoplasma gondii infection in mice. Infect. Immun. 2012;80:2121–32. doi: 10.1128/IAI.06295-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Okomo-Adhiambo M, Beattie C, Rink A. cDNA microarray analysis of host-pathogen interactions in a porcine in vitro model for Toxoplasma gondii infection. Infect. Immun. 2006;74:4254–65. doi: 10.1128/IAI.00386-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teo CF, Zhou XW, Bogyo M, Carruthers VB. Cysteine protease inhibitors block Toxoplasma gondii microneme secretion and cell invasion. Antimicrob. Agents Chemother. 2007;51:679–88. doi: 10.1128/AAC.01059-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Infection of BeWo cells by T. gondii and treatments


