Figure 5.
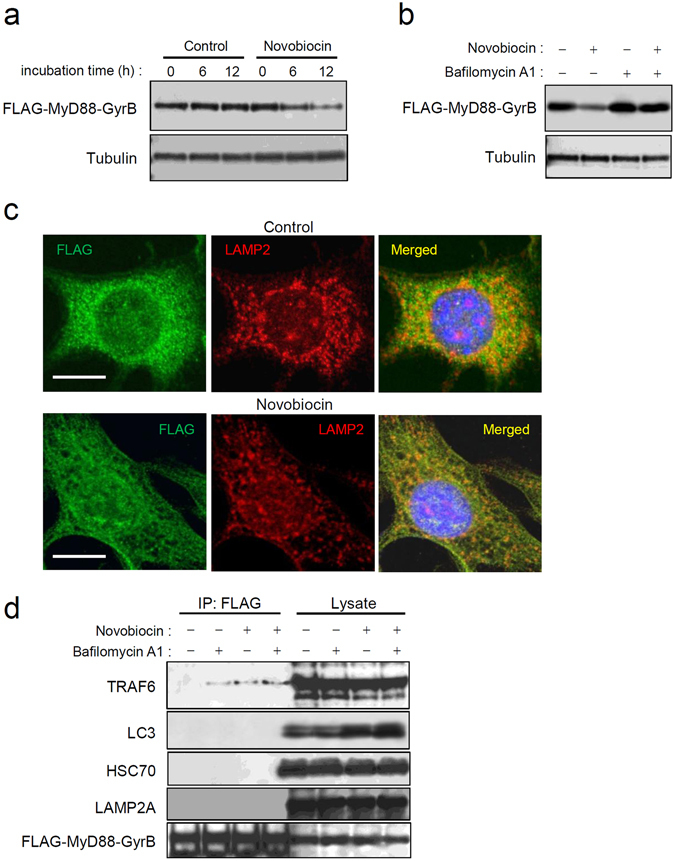
Monomeric MyD88 is targeted by basal autophagy. (a) Monomerization of MyD88-GyrB promotes its degradation. Myd88−/− MEFs stably expressing FLAG-tagged MyD88-GyrB were incubated with 10 μM novobiocin for the indicated periods, followed by cell lysis. Expression levels of FLAG-MyD88-GyrB and α-tubulin were assessed by immunoblotting. All the blots were obtained under the same experimental conditions, and the cropped images of the blots are shown. The uncropped images are in Supplementary Fig. 22. (b) Degradation of monomerized MyD88-GyrB is inhibited by lysosomal inhibition. Myd88−/− MEFs stably expressing FLAG-tagged MyD88-GyrB were incubated with or without 10 μM novobiocin for 12 h in the presence or absence of 100 nM BafA1. Expression levels of FLAG-MyD88-GyrB and α-tubulin were assessed by immunoblotting. All the blots were obtained under the same experimental conditions, and the cropped images of the blots are shown. (c) Speckles of MyD88-GyrB are downregulated by monomerization. Myd88−/− MEFs stably expressing FLAG-tagged MyD88-GyrB were incubated with 0.5% DMSO or 10 μM novobiocin for 12 h. Immunofluorescent staining of the cells for FLAG (green) and LAMP2 (red) was carried out, and cell nuclei were stained with Hoechst 33342. Images were obtained by confocal microscopy. Scale bar: 10 μm. (d) Monomerized MyD88-GyrB interacts with TRAF6. Myd88−/− MEFs stably expressing FLAG-tagged MyD88-GyrB were incubated with or without 10 μM novobiocin for 12 h in the presence or absence of 100 nM BafA1, followed by cell lysis. Then immunoprecipitation (IP) using anti-FLAG-agarose was carried out with clarified lysates, followed by immunoblotting for TRAF6, p62/Sqstm1, LC3, ATG5, HSC70, LAMP2A, and FLAG-MyD88-GyrB. All the blots were obtained under the same experimental conditions, and the cropped images of the blots are shown.
