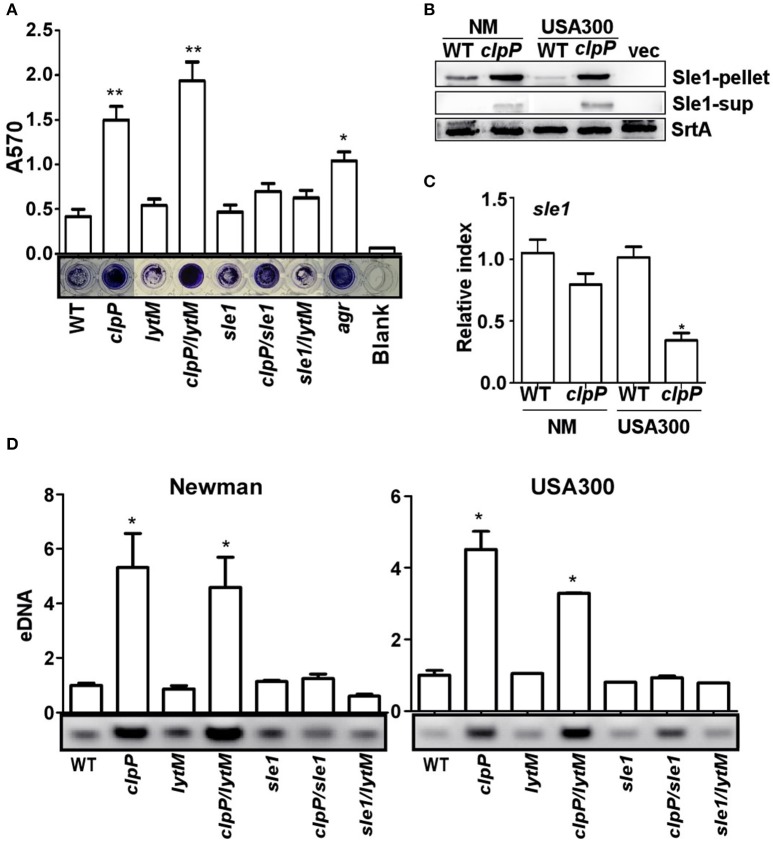
Figure 4.
The overproduction of the cell wall hydrolase Sle1 is responsible for the increased release of eDNA. (A) Biofilm formation was determined in sle1, lytM, clpP/sle1, clpP/lytM double mutant strains in comparison to NM strain (WT) in vitro by semi-quantitative biofilm assay. agr mutant strain was used as a positive control. The density of crystal violet-stained biofilms was measured at 570 nm. Data were derived from three independent experiments, with four replicates of each experiment. *P < 0.05; **P < 0.01 (vs. WT). (B) The expression of the Sle1 protein was determined in samples prepared from stationary-phase (OD600 = 2) cells grown in TSB by Western blot. The wild-type (WT) and clpP mutant strains were used for the assay. Sle1-pellet, Sle1 protein detected in the cell pellets; Sle1-sup, Sle1 protein detected in the supernatant by TCA precipitation; SrtA, Sortase A was used as a loading control. (C) The expression of the sle1 gene was determined in samples prepared from stationary-phase (OD600 = 2) cells grown in TSB, using quantitative RT-PCR. The wild-type (WT) and clpP mutant strains were used for the assay. Data were derived from three biological repeats. *P < 0.05 (vs. WT). (D) Agarose gel of isolated eDNAs. Culture supernatants from the wild-type (WT) and its derivatives were filter sterilized, extracted with phenol-chloroform and ethanol precipitated. Precipitates were suspended in H2O normalized according to OD600 of the initial cultures. Data were derived from three biological repeats. *P < 0.05(vs. WT).