Abstract
Structural colours in living organisms have been observed and analysed in a large number of species, however the study of how the micro- and nano-scopic natural structures responsible of such colourations develop has been largely ignored. Understanding the interplay between chemical composition, structural morphology on multiple length scales, and mechanical constraints requires a range of investigation tools able to capture the different aspects of natural hierarchical architectures. Here, we report a developmental study of the most widespread strategy for structural colouration in nature: the cuticular multilayer. In particular, we focus on the exoskeletal growth of the dock leaf beetle Gastrophysa viridula, capturing all aspects of its formation: the macroscopic growth is tracked via synchrotron microtomography, while the submicron features are revealed by electron microscopy and light spectroscopy combined with numerical modelling. In particular, we observe that the two main factors driving the formation of the colour-producing multilayers are the polymerization of melanin during the ecdysis and the change in the layer spacing during the sclerotisation of the cuticle. Our understanding of the exoskeleton formation provides a unique insight into the different processes involved during metamorphosis.
Introduction
In contrast to colours due to pigments, structural colouration originates from the interaction of light with sub-micrometer structured materials1. Examples of such striking and brilliant colourations are found in many different groups of organisms, including bacteria2, protists3, plants4, and animals5. The variety of colour-producing photonic mechanisms is tremendous - ranging from one- to three-dimensional systems. These structures can be highly periodic (where the long-range correlations between the elements give rise to bright metallic colourations6), partially disordered (here, the short-range correlations allow matte, isotropic colours7), or completely random (the absence of correlation between the scattering centres provides brilliant white reflectivity8, 9). Having evolved over more than 500 million years10–12, structural colours have been proven to play a key role in animal communication13, mating14, and camouflage15.
Such photonic structures are frequently found among arthropods and are particularly common in insects16. In fact, a great number of different species show structurally coloured scales15 or setae17. The most common and best understood colour-producing mechanism by far is the multilayer reflector, which is often found in beetles (Coleoptera). These reflectors may be located at different depths within the cuticle18, 19, which forms a multilayered exoskeleton20.
Being a natural fibre composite, the insect cuticle consists of chitin microfibrils embedded in a proteinous matrix21, 22 which usually contains pigments such as tannins or melanin. In many iridescent insects, multilayer reflectors are generated by stratified deposition of pigments in different cuticular layers. Depending on the pigments types and contents, the refractive index of pigmented layers can vary significantly with respect to the chitin-protein matrix23.
It has long been known that the cuticle is secreted by a single-sheet epithelium24 whose products can vary in time during the development, giving overlying layers: the outermost epicuticle and the underlying, thicker procuticle, which can be further divided into exocuticle and endocuticle21. The development of the trabeculae (i.e. the columns connecting top and bottom sides of the elytra) has also been investigated and reported to begin from the dorsal cuticle and progress towards the ventral cuticle and eventually merge with it25. However, little is known about the development of the photonic structures: previous studies have concentrated on the development of butterfly scales26–29 and bird feathers barbs30 showing the interplay of self-assembly and biologically-driven development. Even though the literature on metallic colouration in beetles is extensive, describing standard multilayer reflectors31, circularly polarising helicoids32, chirped broadband reflectors18, and their taxonomic distribution33, 34, their formation and development has never been investigated in living specimens. Employing a hierarchical approach, we describe the formation of a multilayer reflector in the exocuticle of developing European green dock leaf beetles (Gastrophysa viridula): from the observation of the macroscopic features to the investigation of micro-sized architectures via synchrotron microtomography (SR-μCT) down to the nanoscale imaged by transmission electron microscopy (TEM). To complement the results, optical characterization of the developing exoskeleton is carried out on the cuticle of living specimens. The collected spectra for different stages of growth are then compared to computational simulations based on the transfer-matrix method35.
Results and Discussion
The life cycle of G. viridula generally consists of the following phases: egg, three larval stages, pupa, and imago. In this study, we focus on the formation of the cuticular multilayer reflector in the hardened forewings (elytra) of the maturing adult beetle and refer to the following stages in the text: egg (1), third instar larva (2), young pupa (3), imago immediately after ecdysis (4), imago before cuticular expansion is completed (5), imago with fully developed cuticle (6). For a summary of the stages and relative imaging techniques, refer to Table 1.
Table 1.
Summary of beetle’s life stages considered in this study.
| Description | Age (/days) | Photo | SR-μCT | TEM | Micrograph | Spectrum |
|---|---|---|---|---|---|---|
| 1. Egg | 0–7 | 1A | ||||
| 2. Third instar larva | 15–18 | 1B | 2A, F | |||
| 3. Young pupa | 18–20 | 1C | 2B, G | 4A | 5A | |
| 4. Imago immediately after ecdysis | 25–26 | 1D | 2C, H | 3A | 4B | 5A, B |
| 5. Imago before cuticular expansion | 26–27 | 1E | 2D, I | 3B | 4C | 5A, C |
| 6. Imago with fully developed cuticle | 30+ | 1F | 2E, J | 3C–F | 4D | 5A, D |
The eggs (Fig. 1A) hatch 5–7 days after deposition. After feeding on dock leaves (Rumex obtusifolius) for 9–10 days, the larvae (Fig. 1B) molt and turn into yellow pupae (Fig. 1C, Supplementary Video 1) from which the imagoes emerge after 5–7 days of metamorphosis (Supplementary Video 2). The lifespan of the adult beetles varies between 20 and 30 days. Immediately after ecdysis, the cuticle of the imagoes is still largely yellow (Fig. 1D) and even one week later, the insects’ cuticles are not fully expanded (Fig. 1E) resulting in a weaker colouration compared to those of older adults (Fig. 1F).
Figure 1.
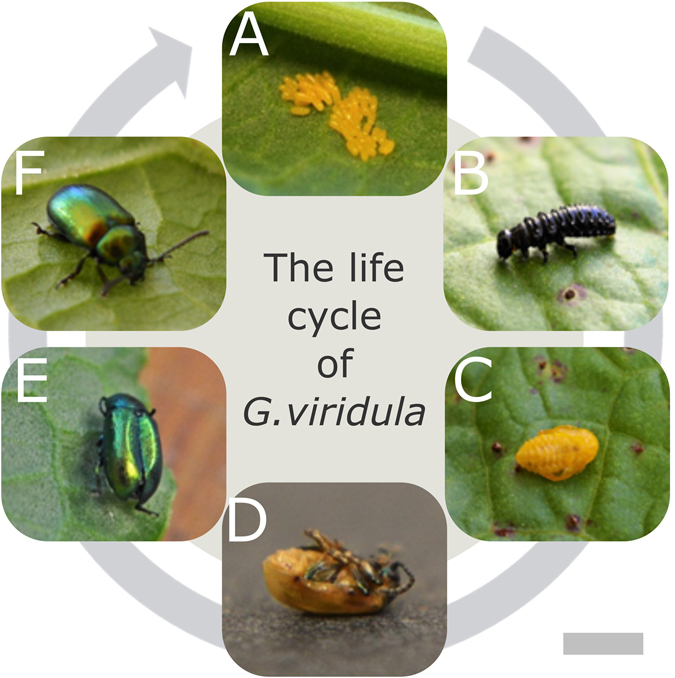
The life cycle of the G. viridula from the egg ((A) day 0–7) to the adult (F) via the larval stages ((B) days 8–18:), pupa ((C) days 18–25), after ecdysis ((D) day 25), and the final cuticle expansion (from (E) day 26). Scale bar: 1 cm.
In order to anatomically characterize the development of the beetles, we perform SR-μCT scans of selected developmental stages (Fig. 2). The larvae do not exhibit wings but rather a soft, non-sclerotised cuticle (Fig. 2a,f). The elytra begin to form in stage 3 (Fig. 2b) and continue during stages 4 and 5 (Fig. 2c,d). However, the cuticle in stage 4 (Fig. 2h) is still considerably softer and thinner (1.0–1.5 μm) than in the adults (4.0–4.5 μm) in stages 5 and 6 (Fig. 2g,j). In stage 4, the trabeculae are seen to originate dorsally (Fig. 2h). It is only at a later stage that the trabeculae start merging with the ventral cuticle (Fig. 2i). Confirming the results in ref. 25, they are seen to have completely developed and thickened in the adult (Fig. 2j), spacing the inner and outer sections of the cuticle, which is about 4 μm thick. Finally, in the fully developed adults (Fig. 2e) the cuticle is completely expanded (Fig. 2j).
Figure 2.
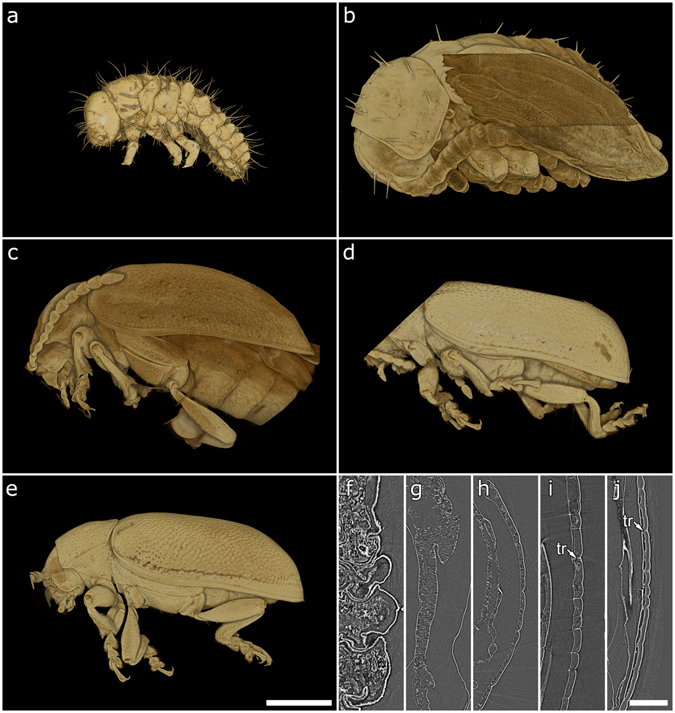
Results from SR-μCT scans showing volume renderings (a–e) and cross-sections (f–j) of the respective dataset. 3rd instar larva, which exhibits a soft cuticle (a,f); young pupa (b,g), pupal skin partly digitally removed to reveal the developing elytron; imago immediately after ecdysis (c,h) the young imago (d,i), which shows a fully-formed yet rucked up cuticle and the formation of trabeculae (“tr” in the figure); old imago (e,j) with a fully developed cuticle. Scale bars: 1 mm.
In order to resolve the cuticular ultrastructure, we image the last stages of the development using TEM and examine an individual immediately after ecdysis (stage 4), a young imago (stage 5; 5 days after emergence), and a fully developed imago (stage 6; 10 days after emergence). In stage 4 the dorsal elytral cuticle has an overall thickness of 1.0–1.5 μm (Fig. 3a). The presumptive epicuticle appears to be formed by two thin layers with the innermost being more highly electron absorbing, (darker in the TEM images) than the outer one. The exocuticle consists of two distinguishable parts: an inner section of tightly packed layers arranged in a helicoidal fashion and outer section showing 5–7 alternating layers of varying electron densities, which constitute the multilayer reflector. At this stage, the endocuticle has not been deposited yet. The outer exocuticle in stage 5 appears thicker and more uniform (Fig. 3b) consisting of 15–20 alternating layers. The contrast between the alternating layers of the reflector is more pronounced. In Fig. 3d,e it is possible to observe a helicoidal organization of the chitinous fibres in the dark-contrasted area of the inner exocuticle. Furthermore, the first endocuticlar layers have been deposited. In stage 6, (Fig. 3c) the arrangement is similar to the earlier stage and only an increase in the number of endocuticular layers is observed. As far as the architecture of the chitin fibrils in cuticle is concerned, we distinguish two types of arrangements: pseudo-orthogonal endocuticular layers (Fig. 3f) and a helicoidal exocuticle (Fig. 3d,e). The pseudo-orthogonal one has been previously reported to be common in beetle endocuticle36–38 but does not exhibit macrofibres, as present in several beetle species22. In the helicoid we can recognize alternating layers of different electron density.
Figure 3.
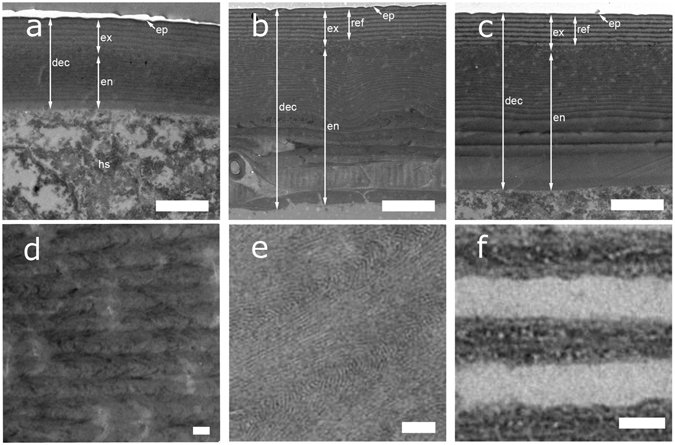
Figure 3 TEM sections of the developing dorsal elytral cuticle (“dec” in the figure), which illustrate the development of the endocuticle (“en”), exocuticle (“ex”), and epicuticle (“ep”). The cuticle after ecdysis (Stage 4: (a)) is considerably thinner than in the later stages 5 and 6 (b,c). The hemolymph space (“hs”) is visibile underneath the elytra. In (b), the young imago shows a more defined exocuticular multilayer reflector (“ref”) and a thicker endocuticle. The old imago, (c) exhibits a fully-formed cuticle. At higher magnification, it is possible to appreciate the structure of the inner exocuticle in (d,e) and the alternating fibril arrangement in the multilayer reflector in (f) for the fully formed imago. Scale bar: 2 μm for (a–c), and 100 nm for (d–f).
The development of the exocuticlar ultrastructure is monitored in living beetles spectroscopically. Figure 4a shows a micrograph in epi-illumination of the young pupal skin and the cuticle of the beetle in stage 4, 5, 6 is illustrated in Fig. 4b–d respectively. The spectral response for stages 3 to 6 is reported in Fig. 5a. By comparing the measured optical response with the one predicted from transfer-matrix calculations35 (Fig. 5a), we can quantify the variations of the cuticle in terms of its composition.
Figure 4.
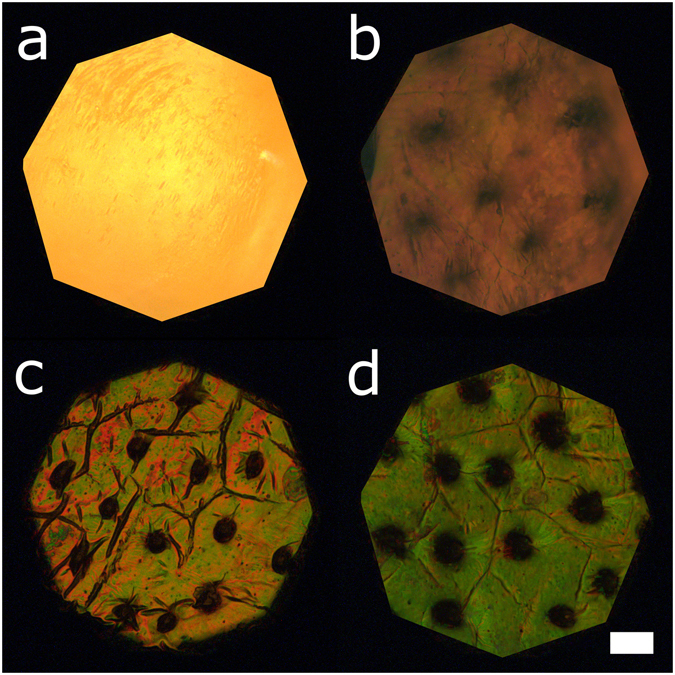
Micrographs of the developing cuticle. (a) shows the yellow pupal skin encasing the imago during pupation, when the cuticle is not yet formed. 3–4 days later (b), the adult cuticle is starting to form and is distinguishable underneath the pupal skin. The formation of trabeculae is evident from the presence of dark invaginations. In (c), the imago has emerged but the cuticle is still not fully expanded. 5 days after ecdysis (d), the cuticle has reached its final conformation. Scale bar: 1 mm.
Figure 5.
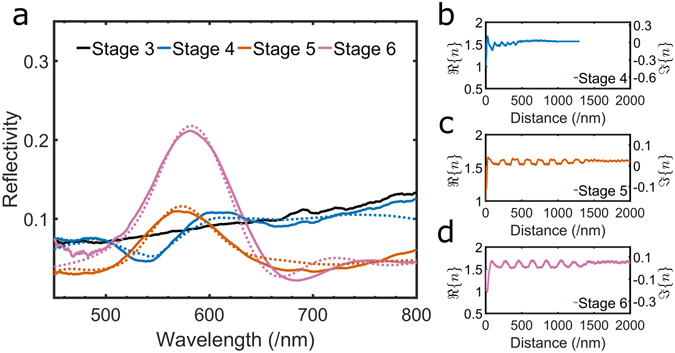
(a) The experimental spectra collected from a 10 μm spot (solid lines) compared to the spectral response as predicted by the transfer-matrix simulations (dashed lines). (b–d) The real part and imaginary part of the refractive index as a function of the cuticle’s depth at different stages of development of the cuticle, see legend.
The independent variables used in transfer-matrix method are: (i) the dimensions of the layers constituting the exocuticle and (ii) their corresponding refractive indexes. To evaluate (i) for each stage of growth we extract the thickness of the layers from the TEM sections (see Supplementary Information). The refractive index of the chitin-protein matrix n 1 used for this study is the one measured in refs 39, 40 for all the stages while the refractive index of the pigmented layers n 2 is calculated using the effective medium approximation for each stage, since the ratio between the chitin-protein matrix and pigments changes during the development, by least squares minimisation of the difference between the predicted and experimental reflectivity. The value of real and imaginary part of the refractive indexes are shown in Fig. 5b–d. In the adult stage we assume that the only pigment present in the exocuticle is melanin, as reported in ref. 41, while during ecdysis a mixture of quinones and melanin is also considered (see Supplementary Information for the full discussion).
The young pupal skin (Fig. 4a) does not show any evidence of structural colour as its spectrum is typical for pigment absorption (Fig. 5a). Nevertheless, this incoherent pigmentary layer is still present immediately before the ecdysis (Fig. 4b) and it plays a filtering role in the measured spectrum42. By absorbing in the 400–500 nm region, the pupal skin decreases the signal produced by the developing multilayer. Hence, the reflectivity in this part of the spectrum is lower than the one predicted from the modelling of the Bragg stack only (Fig. S2).
After ecdysis, we observe a strong drop in reflectivity in the 530–550 nm wavelength region (Fig. 5a) which can be explained including in the calculation the presence of quinones in addition to melanin. These compounds have been shown to play a role in melanogenesis (which takes place during the sclerotisation of the cuticle, as reported in refs 43–45). The presence of quinones is further confirmed by the reddish colouration observed in the microscopic image of the cuticle in stage 4 (Fig. 4b), as expected in presence of this class of compounds46, 47. In particular, we conclude by fitting the measured spectra with our model that the relative amount of melanin and quinones in the pigmented layers in stage 4 is 15% and 5%, respectively. By comparing stage 4 with 5 and 6, we also note that the multilayer is yet not fully developed at this stage: by plotting the complex refractive index as a function of the multilayer’s depth, we observe that the contrast between the layers is low and that the distance between them is small (Fig. 5b–d).
In stage 5 the cuticle shows a broad reflectivity peak around 580 nm (Fig. 5a). At this stage, quinones are not present and the measured spectra agrees with the calculations using a melanin content of 57% for the pigmented layers. Moreover, the layering is very well defined and the distance between layers has substantially increased with respect the other stages (Fig. 5c).
Finally, when the cuticle of the old imago is fully expanded (Fig. 4d), the peak reflectivity is considerably stronger than the one of the younger imago (Fig. 5a). At this stage the melanisation has completed and we estimate that the final percentage of melanin contained in the pigmented layers of chitin-protein matrix is 79% (Fig. 5d).
Conclusion
Our multi-scale imaging and spectroscopical study allowed us to reveal the different processes involved in the development of the exocuticule of a structurally coloured beetle.
We observed that the adult colouration of this species is achieved by an increase in the dimensions of the layers and by their melanisation in the outer exocuticle. Interestingly, the layer deposition and the melanisation happen in different stages. It is particularly surprising that the latter process is observed after stage 4 when the deposition of the endocuticle has already started. Therefore, even small variations during the development can result in extreme colour differences, making these architectures suitable for quick adaptation and species diversification - possibly faster than changing highly conserved biosynthetic pathways for pigmentation, which are controlled by a complex set of enzymes and genetics48, including different gene classes for biosynthesis and spatiotemporal positioning of pigments49. In comparison, altering cuticle deposition patterns appears to be a simpler approach. This is exemplified by the fact of numerous repeated evolutionary origins of multilayer reflectors in beetles18 and also explains how closely related species, such as in the beetle genera Eupholus and Cetonia, can produce very different colourations.
High resolution TEM images reveal that a helicoidal organization of chitin fibrils is maintained in the entire cuticle37. However, even if the characteristic dimensions of such helicoidal architecture do not contribute to the optical response in this case, its dimensions (of about 800 nm) might indicate that exocuticle is predisposed to the development of chiral Bragg reflectors18.
More generally, this study shed new insight on cuticle development: from an evolutionary point of view, it is interesting to note that pigmented-based multilayer reflectors are very common among different insects and other arthropods and therefore the mechanisms observed in this case of development might be generalized to other species, since the same materials/strategies are involved.
Methods
Adult G. viridula beetles were collected from the meadows by the river Cam in Cambridge, England (52°12′41.2″N, 0°07′43.4″E) and reared in the lab as described in ref. 50.
Synchrotron microtomography
Dead beetles at different stages were immersed in an EtOH 70% aqueous solution and scanned at the TOPO-TOMO beamline51 of the ANKA Synchrotron Radiation Facility at KIT, Germany. 3,000 radiographic projections covering an angular range of 180° were acquired using a filtered polychromatic beam with the spectral peak at about 15 keV. An indirect detector system composed of a 12 μm LSO:Tb scintillator52, diffraction limited optical microscope (Optique Peter) and 12 bit pco.dimax high speed camera with 2016 × 2016 pixels resolution53 was employed to capture the frames with an exposure time of 16.6 ms each, resulting in an overall scan duration of 49.8s. A 5x optical magnification led to an effective pixel size of 2.44 μm. Tomographic reconstruction was performed with the GPU-accelerated filtered back projection algorithm implemented in the software framework UFO54. Volume renderings of tomographic data were performed with Drishti 2.5.
Transmission electron microscopy
TEM sections were prepared according to the following procedure: first of all, fixation was initiated by immersing the elytra in a buffer containing glutaraldehyde (2 wt%), formaldehyde (2 wt%) and sodium cacodylate (0.05 M) at pH 7.4 for 18 hours at 4 °C. Then the elytra were rinsed five times with deionized water (DIW) and fixed for 48 hours at 4 °C in a second buffer containing osmium ferricyanide (1 wt%) and sodium cacodylate (0.05 M) at pH 7.4. Next, they were rinsed again in DIW and dehydrated in an ascending series of ethanol solutions from 50 wt% to 100 wt% dry ethanol. The next step was to bulk stain them with magnesium uranyl acetate (3 wt%) in pure dry ethanol for 48 hours at 4 °C in the dark. Afterwards, the elytra were rinsed 5 times in pure dry ethanol and repeatedly frozen and thawed in liquid nitrogen. This procedure was repeated 10 times in order to facilitate the subsequent infiltration with epoxy resin. The elytra were rinsed 5 times in dry ethanol (100 wt%), twice in dry acetone and 3 times in dry acetonitrile. They were incubated overnight in a 50/50 mixture of acetonitrile and Quetol 651 epoxy resin (without the catalyst BDMA) in uncapped tubes to allow the acetonitrile to gradually evaporate. They were subsequently placed in fresh Quetol (without BDMA) every day for 2 weeks and subsequently in Quetol 651 (with BDMA) every day for a further 2 weeks. In order to perform vertical sectioning, the elytra were orientated in a coffin mould and the resin was left to cure for a minimum of 48 hours at 65 °C. Finally, the elytra were sectioned at 50–60 nm with a Leica UCT ultramicrotome using a 35° wedge angle diamond knife (Diatome Ltd) and mounted on 300 mesh copper grids with a carbon film (EM Resolutions Ltd) for imaging. They were viewed in a FEI Tecnai G2 operated at 200 kV (camera: AMT XR60B; software: Deben).
Optical microspectroscopy
Optical microscopy was performed using a Zeiss Axio.Scope optical microscope in Köhler illumination equipped with a 20X objective (Zeiss EC Epiplan-APOCHROMAT 0.6 HD DIC) coupled to a spectrometer (Avantes HS2048) via an optical fibre (Thorlabs, FC-UV100-2-SR). This enabled us to collect the spectra from a circular spot (diameter: 10 μm). 20 spectra were collected for each sample using an integration time of 500 ms and normalized with respect to a protected silver mirror (Thorlabs, PF10-03-P01). These experimental spectra were then compared to those originated from a transfer-matrix simulation. The simulation was based on the open source Python code developed in ref. 55. Both the real and imaginary part of the refractive index were assumed to be proportional to the optical density derived from the TEM images by converting the multilayer image to grey levels averaged using ImageJ56. The TEM images were rescaled as described in the Supplementary Information in order to account for the small deformations that occurred during the sample preparation. The refractive indices accounted for dispersion. 20 such profiles with a width of 500 nm were used as input for the model which was run for light incident from −37° to 37°. The spectra obtained were then averaged together. In this way, we replicated the experimental conditions in which the spectra were then collected from a 10 μm spot with a numerical aperture of 0.6.
Data availability
All the research data supporting the publication are available from the University of Cambridge data repository (https://doi.org/10.17863/CAM.8829).
Electronic supplementary material
Acknowledgements
Yanmin Zhou and Walter Federle for the advice on beetle rearing and Giulia Guidetti for continuous TLC; Bodo D. Wilts and Villads E. Johansen guidance on numerical methods; Hartmut Greven for advice. This work was supported by the BBSRC David Phillips fellowship [BB/K014617/1], and the ERC-2014-STG H2020 639088 SeSaMe. Research at KIT was partially funded by the German Federal Ministry of Education and Research by grants 05K10CKB and 05K12CK2.
Author Contributions
S.V., O.D.O., T.v.d.K. designed research; O.D.O., T.v.d.K., J.N.S., J.P., T.d.S.R., T.B. performed research, O.D.O. and T.v.d.K. analyzed data; O.D.O., T.v.d.K., and S.V. wrote the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-01496-8
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kinoshita S, Yoshioka S, Miyazaki J. Physics of structural colors. Reports on Progress in Physics. 2008;71:076401. doi: 10.1088/0034-4885/71/7/076401. [DOI] [Google Scholar]
- 2.Kientz B, Vukusic P, Luke S, Rosenfeld E. Iridescence of a Marine Bacterium and Classification of Prokaryotic Structural Colors. Applied and Environmental Microbiology. 2012;78:2092. doi: 10.1128/AEM.07339-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon R, Losic D, Tiffany MA, Nagy SS, Sterrenburg FA. The Glass Menagerie: diatoms for novel applications in nanotechnology. Trends in Biotechnology. 2009;27:116. doi: 10.1016/j.tibtech.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Vignolini S, Moyroud E, Glover BJ, Steiner U. Analysing photonic structures in plants. Journal of The Royal Society Interface. 2013;10:20130394. doi: 10.1098/rsif.2013.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vukusic P, Sambles JR. Photonic structures in biology. Nature. 2003;424:852. doi: 10.1038/nature01941. [DOI] [PubMed] [Google Scholar]
- 6.Vukusic P, Sambles JR, Lawrence CR, Wootton RJ. Quantified interference and diffraction in single Morpho butterfly scales. Proceedings of the Royal Society B: Biological Sciences. 1999;266:1403. doi: 10.1098/rspb.1999.0794. [DOI] [Google Scholar]
- 7.Noh, H. et al. Structural Color: How Noniridescent Colors Are Generated by Quasi-ordered Structures of Bird Feathers (Adv. Mater. 26–27/2010). Advanced Materials22, n/a (2010). [DOI] [PubMed]
- 8.Vukusic P, Hallam B, Noyes J. Brilliant Whiteness in Ultrathin Beetle Scales. Science. 2007;315:348. doi: 10.1126/science.1134666. [DOI] [PubMed] [Google Scholar]
- 9.Burresi, M. et al. Bright-White Beetle Scales Optimise Multiple Scattering of Light. Scientific Reports4, 10.1038/srep06075 (2014). [DOI] [PMC free article] [PubMed]
- 10.Parker AR. 515 million years of structural colour. Journal of Optics A: Pure and Applied Optics. 2000;2:R15–R28. doi: 10.1088/1464-4258/2/6/201. [DOI] [Google Scholar]
- 11.Vinther J, Briggs DEG, Clarke J, Mayr G, Prum RO. Structural coloration in a fossil feather. Biology Letters. 2009;6:128. doi: 10.1098/rsbl.2009.0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNamara ME, Briggs DEG, Orr PJ, Noh H, Cao H. The original colours of fossil beetles. Proceedings of the Royal Society B: Biological Sciences. 2011;279:1114. doi: 10.1098/rspb.2011.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemp DJ, Herberstein ME, Grether GF. Unraveling the true complexity of costly color signaling. Behavioral Ecology. 2011;23:233. doi: 10.1093/beheco/arr153. [DOI] [Google Scholar]
- 14.Loyau A, Gomez D, Moureau B, Théry M, Hart NS. Iridescent structurally based coloration of eyespots correlates with mating success in the peacock. Behavioral Ecology. 2007;18:1123. doi: 10.1093/beheco/arm088. [DOI] [Google Scholar]
- 15.Wilts BD, Michielsen K, Kuipers J, De Raedt H, Stavenga DG. Brilliant camouflage: photonic crystals in the diamond weevil, Entimus imperialis. Proceedings of the Royal Society B: Biological Sciences. 2012;279:2524. doi: 10.1098/rspb.2011.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saranathan V, Seago AE, Sandy A, Narayanan S. Structural diversity of arthropod biophotonic nanostructures spans amphiphilic phase-space. Nano Letters. 2015;15:3735. doi: 10.1021/acs.nanolett.5b00201. [DOI] [PubMed] [Google Scholar]
- 17.Trzeciak TM, Vukusic P. Photonic crystal fiber in the polychaete worm Pherusa sp. Phys. Rev. E. 2009;80:061908. doi: 10.1103/PhysRevE.80.061908. [DOI] [PubMed] [Google Scholar]
- 18.Seago AE, Brady P, Vigneron JP, Schultz TD. Gold bugs and beyond: a review of iridescence and structural colour mechanisms in beetles (Coleoptera) Journal of The Royal Society Interface. 2008;6:S165–S184. doi: 10.1098/rsif.2008.0354.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasteels JM, Deparis O, Mouchet SR, Windsor DM, Billen J. Structural and physical evidence for an endocuticular gold reflector in the tortoise beetle, Charidotella ambita. Arthropod Structure & Development. 2016;45:509. doi: 10.1016/j.asd.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Kristensen N. Vol 2: Morphology, Physiology, and Development. Berlin: De Gruyter; 2003. pp. 23–38. [Google Scholar]
- 21.Neville, A. C. Biology of the Arthropod Cuticle, 160–168 (Springer-Verlag Berlin, Heidelberg, 1975).
- 22.van de Kamp T, Riedel A, Greven H. Micromorphology of the elytral cuticle of beetles, with an emphasis on weevils (Coleoptera: Curculionoidea) Arthropod Structure & Development. 2016;45:14. doi: 10.1016/j.asd.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Stavenga DG, Leertouwer HL, Hariyama T, De Raedt HA, Wilts BD. Sexual Dichromatism of the Damselfly Calopteryx japonica Caused by a Melanin-Chitin Multilayer in the Male Wing Veins. PloS one. 2012;7:e49743. doi: 10.1371/journal.pone.0049743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Picken, L. The Organization of cells and other organisms, 407–487 (Clarendon Press, Oxford, 1962).
- 25.Scherf H. Beiträge zur kenntnis des cuticularpanzers der käfer auf grund von untersuchungen im polarisierten licht an larve, puppe und imago von Oryctes nasicornis L. Zeitschrift für Morphologie und Ökologie der Tiere. 1954;43:213. doi: 10.1007/BF00391844. [DOI] [Google Scholar]
- 26.Ghiradella H. Structure and development of iridescent butterfly scales: Lattices and laminae. Journal of Morphology. 1989;202:69. doi: 10.1002/jmor.1052020106. [DOI] [PubMed] [Google Scholar]
- 27.Ghiradella H. Structure of butterfly scales: patterning in an insect cuticle. Microscopy Research and Technique. 1994;27:429. doi: 10.1002/jemt.1070270509. [DOI] [PubMed] [Google Scholar]
- 28.Ghiradella HT, Butler MW. Many variations on a few themes: a broader look at development of iridescent scales (and feathers) Journal of The Royal Society Interface. 2009;6:S243–S251. doi: 10.1098/rsif.2008.0372.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saranathan V, et al. Structure, function, and self-assembly of single network gyroid (I4132) photonic crystals in butterfly wing scales. Proceedings of the National Academy of Sciences. 2010;107:11676. doi: 10.1073/pnas.0909616107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prum RO, Dufresne ER, Quinn T, Waters K. Development of colour-producing -keratin nanostructures in avian feather barbs. Journal of The Royal Society Interface. 2009;6:S253–S265. doi: 10.1098/rsif.2008.0466.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parker AR. The diversity and implications of animal structural colours. Journal of Experimental Biology. 1998;201:2343–2347. doi: 10.1242/jeb.201.16.2343. [DOI] [PubMed] [Google Scholar]
- 32.Sharma V, Crne M, Park JO, Srinivasarao M. Structural origin of circularly polarized iridescence in jeweled beetles. Science. 2009;325:449. doi: 10.1126/science.1172051. [DOI] [PubMed] [Google Scholar]
- 33.Hunt T, Bergsten J, Levkanicova Z. A comprehensive phylogeny of beetles reveals the evolutionary origins of a superradiation. Science. 2007;318:1913. doi: 10.1126/science.1146954. [DOI] [PubMed] [Google Scholar]
- 34.Del Rio, L. F., Arwin, H. & Järrendahl, K. Polarizing properties and structure of the cuticle of scarab beetles from the Chrysina genus. Physical Review E94 (2016). [DOI] [PubMed]
- 35.Yeh, P. Optical waves in layered media, 118–142 (John Wiley & Sons inc., Hoboken, 2005).
- 36.Neville AC, Luke BM. A Two-system model for chitin-protein complexes in insect cuticles. Tissue and Cell. 1969;1:689. doi: 10.1016/S0040-8166(69)80041-8. [DOI] [PubMed] [Google Scholar]
- 37.Neville AC, Luke BM. Molecular architecture of adult locust cuticle at the electron microscope level. Tissue and Cell. 1969;1:355. doi: 10.1016/S0040-8166(69)80030-3. [DOI] [PubMed] [Google Scholar]
- 38.van de Kamp T, Greven H. On the architecture of beetle elytra. Entomologie heute. 2010;22:191–204. [Google Scholar]
- 39.Leertouwer HL, Wilts BD, Stavenga DG. Refractive index and dispersion of butterfly chitin and bird keratin measured by polarizing interference microscopy. Optics Express. 2011;19:24061. doi: 10.1364/OE.19.024061. [DOI] [PubMed] [Google Scholar]
- 40.Stavenga DG, Leertouwer HL, Wilts BD. Quantifying the refractive index dispersion of a pigmented biological tissue using Jamin–Lebedeff interference microscopy. Light: Science & Applications. 2013;2:e100. doi: 10.1038/lsa.2013.56. [DOI] [Google Scholar]
- 41.Noyes JA, Vukusic P, Hooper IR. Experimental method for reliably establishing the refractive index of buprestid beetle exocuticle. Optics Express. 2007;15:4351. doi: 10.1364/OE.15.004351. [DOI] [PubMed] [Google Scholar]
- 42.Trzeciak TM, Wilts BD, Stavenga DG, Vukusic P. Variable multilayer reflection together with long-pass filtering pigment determines the wing coloration of papilionid butterflies of the nireus group. Optics express. 2012;20:8877–8890. doi: 10.1364/OE.20.008877. [DOI] [PubMed] [Google Scholar]
- 43.Sugumaran M. Complexities of cuticular pigmentation in insects. Pigment Cell & Melanoma Research. 2009;22:523. doi: 10.1111/j.1755-148X.2009.00608.x. [DOI] [PubMed] [Google Scholar]
- 44.Andersen SO. Insect cuticular sclerotization: A review. Insect Biochemistry and Molecular Biology. 2010;40:166. doi: 10.1016/j.ibmb.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Moussian B. Recent advances in understanding mechanisms of insect cuticle differentiation. Insect Biochemistry and Molecular Biology. 2010;40:363. doi: 10.1016/j.ibmb.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Uesawa Y, Mohri K. Degradation of Methyldopa by Banana. Pharmaceuticals. 2010;3:441. doi: 10.3390/ph3030441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inaba K, Takahashi Y, Ito K. Critical role of a thiolate-quinone charge transfer complex and its adduct form in de novo disulfide bond generation by DsbB. Proceedings of the National Academy of Sciences. 2006;103:287. doi: 10.1073/pnas.0507570103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shamim, G., Ranjan, S. K., Pandey, D. M. & Ramani, R. Biochemistry and biosynthesis of insect pigments. European Journal of Entomology, 10.14411/eje.2014.021 (2014).
- 49.Wittkopp PJ, Beldade P. Development and evolution of insect pigmentation: Genetic mechanisms and the potential consequences of pleiotropy. Seminars in Cell & Developmental Biology. 2009;20:65. doi: 10.1016/j.semcdb.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Voigt D, Hosoda N, Schuppert J, Gorb S. On the laboratory rearing of green dock leaf beetles Gastrophysa viridula (Coleoptera: Chrysomelidae) Insect Science. 2011;18:379. doi: 10.1111/j.1744-7917.2010.01355.x. [DOI] [Google Scholar]
- 51.Rack A, et al. The micro-imaging station of the TopoTomo beamline at the ANKA synchrotron light source. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. 2009;267:1978. doi: 10.1016/j.nimb.2009.04.002. [DOI] [Google Scholar]
- 52.Cecilia A, et al. LPE grown LSO:Tb scintillator films for high-resolution X-ray imaging applications at synchrotron light sources. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2011;648:S321–S323. doi: 10.1016/j.nima.2010.10.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.dos Santos Rolo T, Ershov A, Van de Kamp T, Baumbach T. In vivo X-ray cine-tomography for tracking morphological dynamics. Proceedings of the National Academy of Sciences. 2014;111:3921. doi: 10.1073/pnas.1308650111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vogelgesang, M., Chilingaryan, S., dos Santos Rolo, T. & Kopmann, A. Ufo: A scalable gpu-based image processing framework for on-line monitoring. In High Performance Computing and Communication & 2012 IEEE 9th International Conference on Embedded Software and Systems (HPCC-ICESS), 2012 IEEE 14th International Conference on, 824–829 (IEEE, 2012).
- 55.Byrnes, S. J. Multilayer optical calculations. arXiv e-prints, doi:arXiv:1603.02720v2 (2016).
- 56.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 2012;9:671. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the research data supporting the publication are available from the University of Cambridge data repository (https://doi.org/10.17863/CAM.8829).


