Abstract
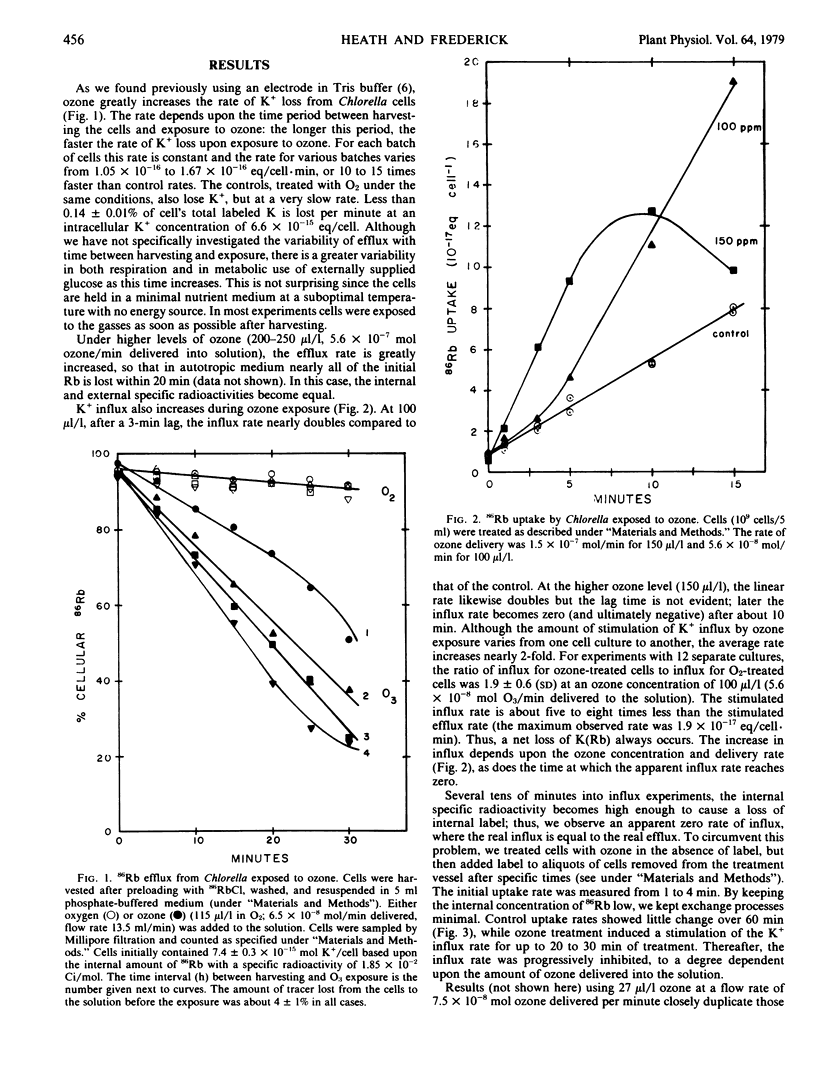
The addition of ozone to a suspension of Chlorella sorokiniana causes a rapid loss of K+, as measured by efflux of 86Rb from prelabeled cells. The efflux of the tracer is stimulated some 15 to 20 times over that of the control. For about 100 microliters per liter ozone, about 25 minutes (6 × 10−8 moles O3 delivered per minute) of exposure are required for a 50% depletion of the intracellular K+. The stimulation of K+ efflux is nearly linearly dependent upon the amount of ozone delivered into the solution. Following short pulses of ozone (lasting 1 to 5 minutes), efflux rates return to the control level but only after about 15 minutes.
While influx of K+ is ultimately inhibited by ozone, at low concentrations or for short exposure times the tracer influex is stimulated 100 to 200%. Ozone stimulation of an active pump mechanism is unlikely in view of a concomitant decrease in respiration. Thus, this influx may represent movement of K+ along its electrochemical gradient. Assuming that influx and efflux are in steady-state according to the Goldman equation, it was calculated that the membrane potential for K+ of −80 to −90 millivolts in control cells drops to −40 millivolts with ozone exposure and is accompanied by a calculated increased permeability to K+ of 2- to 3-fold.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber J. Measurement of the membrane potential and evidence for active transport of ions in Chlorella pyrenoidosa. Biochim Biophys Acta. 1968 Jun 11;150(4):618–625. doi: 10.1016/0005-2736(68)90051-5. [DOI] [PubMed] [Google Scholar]
- Barber J. The efflux of potassium from Chlorella pyrenoidosa. Biochim Biophys Acta. 1968 Dec 10;163(4):531–538. doi: 10.1016/0005-2736(68)90082-5. [DOI] [PubMed] [Google Scholar]
- Barber J. The influx of potassium into Chlorella pyrenoidosa. Biochim Biophys Acta. 1968 Sep 17;163(2):141–149. doi: 10.1016/0005-2736(68)90091-6. [DOI] [PubMed] [Google Scholar]
- Chapman I. V., Sturrock M. G. Radiation-induced decrease in influx rates of potassium ions into thymocytes in vitro in relation to decreased intracellular adenosine triphosphate concentrations. Int J Radiat Biol Relat Stud Phys Chem Med. 1975 Aug;28(2):155–163. doi: 10.1080/09553007514550891. [DOI] [PubMed] [Google Scholar]
- Chimiklis P. E., Heath R. L. Ozone-induced Loss of Intracellular Potassium Ion from Chlorella sorokiniana. Plant Physiol. 1975 Dec;56(6):723–727. doi: 10.1104/pp.56.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick P. E., Heath R. L. Ozone-induced Fatty Acid and Viability Changes in Chlorella. Plant Physiol. 1975 Jan;55(1):15–19. doi: 10.1104/pp.55.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath R. L. Penetration of Mannitol into the Intracellular Space of Chlorella sorokiniana. Plant Physiol. 1977 May;59(5):911–914. doi: 10.1104/pp.59.5.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpas A. B., Baer H., Hooton M. L., Evans R. A high molecular weight allergenic fraction of honeybee venom. J Allergy Clin Immunol. 1977 Sep;60(3):155–162. doi: 10.1016/0091-6749(77)90118-x. [DOI] [PubMed] [Google Scholar]
- Komor E., Tanner W. The determination of the membrane ptoential of Chlorella vulgaris. Evidence for electrogenic sugar transport. Eur J Biochem. 1976 Nov 1;70(1):197–204. doi: 10.1111/j.1432-1033.1976.tb10970.x. [DOI] [PubMed] [Google Scholar]
- Lambert P. A., Hammond S. M. Potassium fluxes, first indications of membrane damage in micro-organisms. Biochem Biophys Res Commun. 1973 Sep 18;54(2):796–799. doi: 10.1016/0006-291x(73)91494-0. [DOI] [PubMed] [Google Scholar]
- Leibowitz M. E., Johnson M. C. Relation of lipid peroxidation to loss of cations trapped in liposomes. J Lipid Res. 1971 Nov;12(6):662–670. [PubMed] [Google Scholar]
- Myers D. K. Some aspects of radiation effects on cell membranes. Adv Biol Med Phys. 1970;13:219–234. doi: 10.1016/b978-0-12-005213-4.50010-7. [DOI] [PubMed] [Google Scholar]
- Nobel P. S., Wang C. T. Ozone increases the permeability of isolated pea chloroplasts. Arch Biochem Biophys. 1973 Aug;157(2):388–394. doi: 10.1016/0003-9861(73)90654-1. [DOI] [PubMed] [Google Scholar]
- Slayman C. L., Long W. S., Lu C. Y. The relationship between ATP and an electrogenic pump in the plasma membrane of Neurospora crassa. J Membr Biol. 1973;14(4):305–338. doi: 10.1007/BF01868083. [DOI] [PubMed] [Google Scholar]


