Abstract
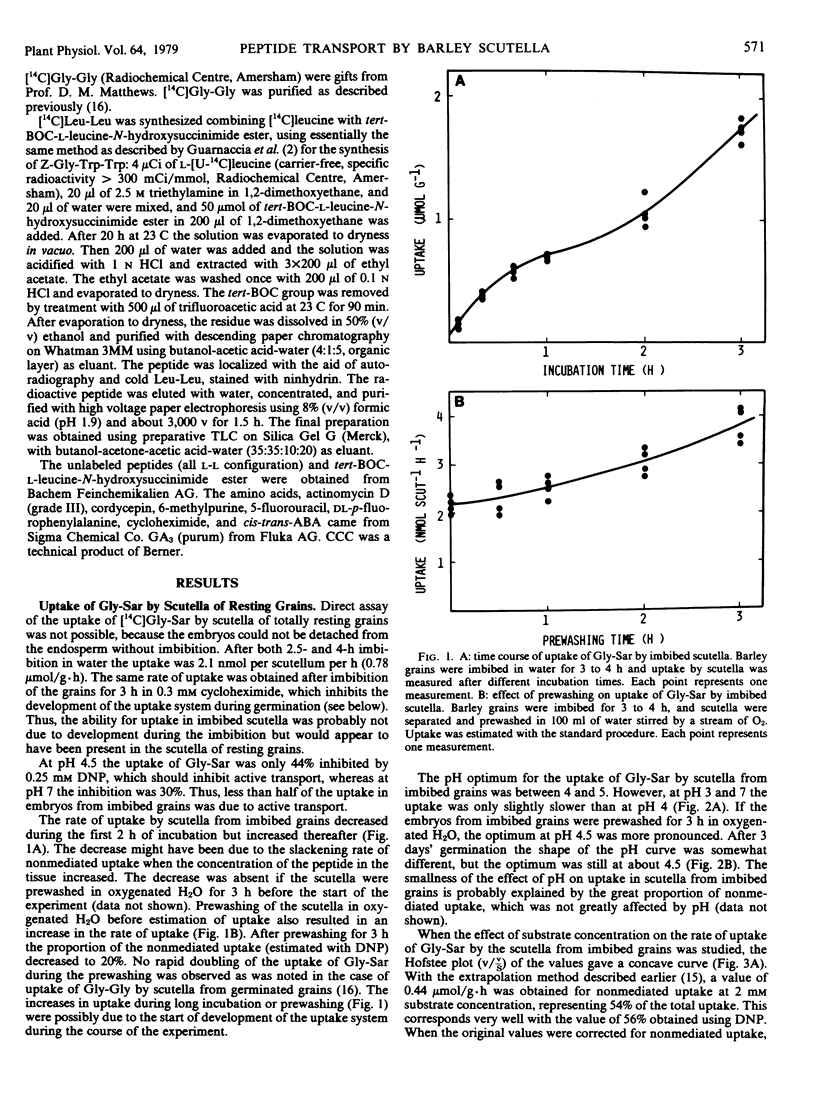
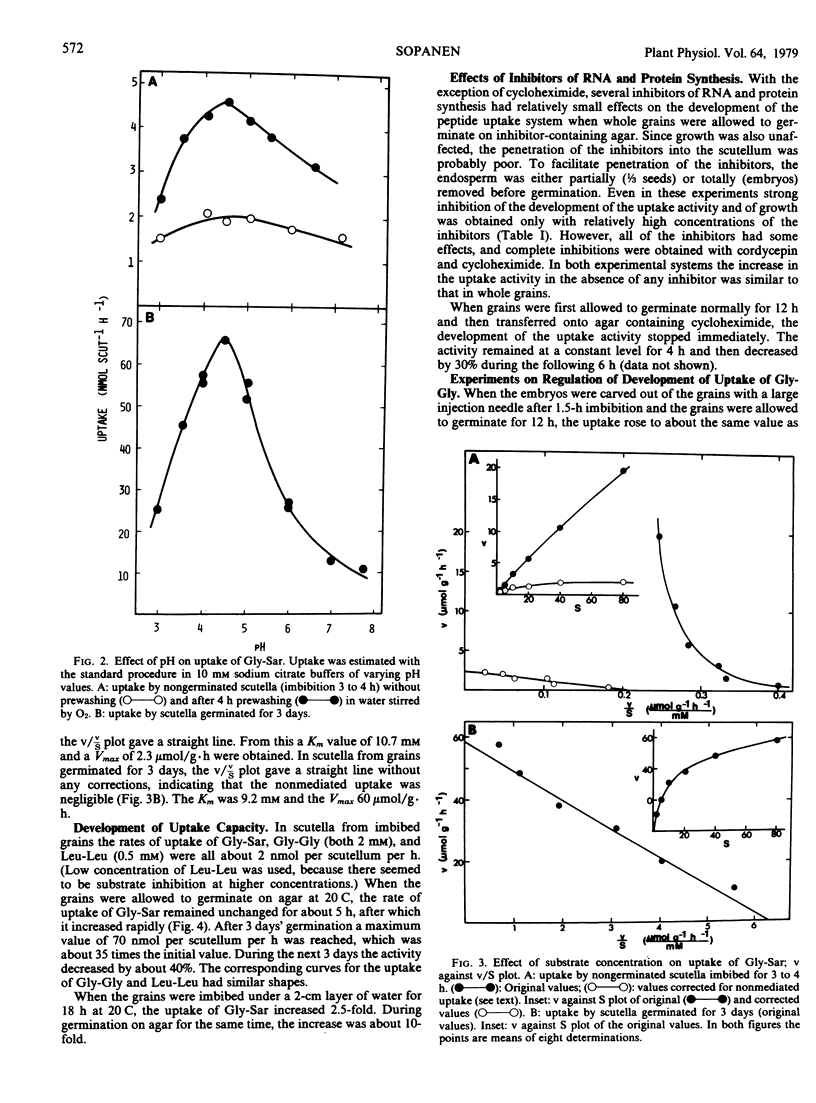
The scutella separated from nongerminated grains of barley (Hordeum vulgare L. cv. Pirkka) took up labeled glycylsarcosine (Gly-Sar), glycylglycine, and leucylleucine at a rate of about 2 nanomoles per scutellum per hour. About 55% of the uptake of Gly-Sar (2 millimolar) was due to nonmediated uptake, but the active component was similar to that operating in the scutella from germinating grains (pH optimum about 4.5, Km about 10 millimolar).
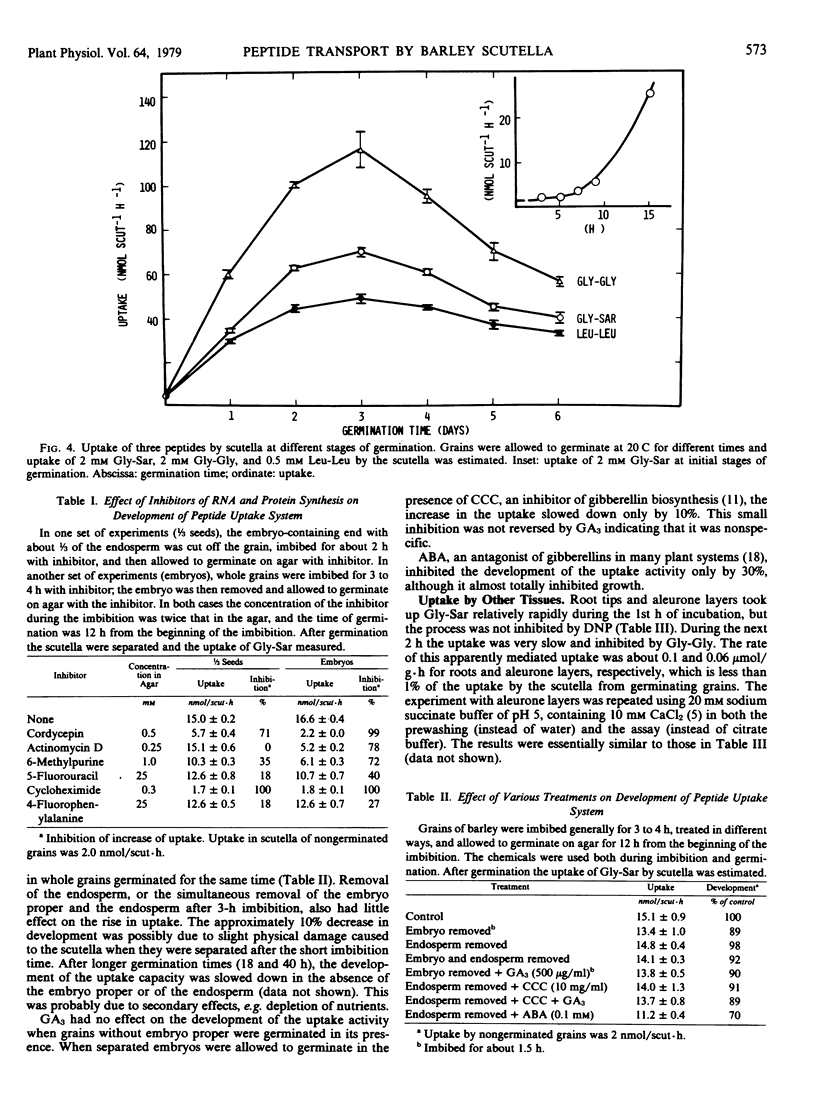
When the grains were allowed to germinate aseptically on agar, the rate of uptake of Gly-Sar began to increase after a lag of about 5 hours. The increase continued until a maximum value of 70 nanomoles per scutellum per hour (35-fold increase) was attained after 3 days of germination. After this the activity began to decrease slowly. The uptake of glycylglycine and leucylleucine changed in a similar way. When embryo-containing halves of seeds or separated embryos were allowed to germinate for 12 hours on agar containing various inhibitors of RNA and protein synthesis, the increase in the peptide uptake activity was completely or partially inhibited. The increase was not affected by removal of the “embryo proper” or endosperm, or by additions of gibberellic or abscisic acid.
Root tips and aleurone layers took up Gly-Sar very slowly; the rate of the mediated component of uptake was less than 1% of the uptake by scutella.
The preferential localization of the peptide transport system in the scutellum and its rapid development at the beginning of germination strengthen the earlier conclusion that peptide transport plays an essential role in the mobilization of reserve proteins during germination.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Enari T. M., Mikola J. Peptidases in germinating barley grain: properties, localization and possible functions. Ciba Found Symp. 1977;(50):335–352. [PubMed] [Google Scholar]
- Ho T. D., Varner J. E. Density labeling of proteins with 13C-labeled amino acids: no accumulation of an inactive alpha-amylase precursor in barley aleurone cells. Arch Biochem Biophys. 1978 Apr 30;187(2):441–446. doi: 10.1016/0003-9861(78)90055-3. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Dure L. S., 3rd The developmental biochemistry of cottonseed embryogenesis and germination. 3. Regulation of the biosynthesis of enzymes utilized in germination. J Biol Chem. 1972 Aug 25;247(16):5048–5055. [PubMed] [Google Scholar]
- Jacobsen J. V., Varner J. E. Gibberellic Acid-induced synthesis of protease by isolated aleurone layers of barley. Plant Physiol. 1967 Nov;42(11):1596–1600. doi: 10.1104/pp.42.11.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxender D. L. Membrane transport. Annu Rev Biochem. 1972;41(10):777–814. doi: 10.1146/annurev.bi.41.070172.004021. [DOI] [PubMed] [Google Scholar]
- Schroeder R. L., Burger W. C. Development and Localization of Carboxypeptidase Activity in Embryo-less Barley Half-kernels. Plant Physiol. 1978 Sep;62(3):458–462. doi: 10.1104/pp.62.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopanen T., Burston D., Matthews D. M. Uptake of small peptides by the scutellum of germinating barley. FEBS Lett. 1977 Jul 1;79(1):4–7. doi: 10.1016/0014-5793(77)80337-2. [DOI] [PubMed] [Google Scholar]
- Sopanen T., Burston D., Taylor E., Matthews D. M. Uptake of glycylglycine by the scutellum of germinating barley grain. Plant Physiol. 1978 Apr;61(4):630–633. doi: 10.1104/pp.61.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visuri K., Mikola J., Enari T. M. Isolation and partial characterization of a carboxypeptidase from barley. Eur J Biochem. 1969 Jan;7(2):193–199. doi: 10.1111/j.1432-1033.1969.tb19591.x. [DOI] [PubMed] [Google Scholar]
- Wiegman T., Woldring M. G., Pratt J. J. A new cocktail for liquid scintillation counting of aqueous radioimmunoassay-samples. Clin Chim Acta. 1975 Mar 24;59(3):347–356. doi: 10.1016/0009-8981(75)90010-8. [DOI] [PubMed] [Google Scholar]


