Abstract
Background:
Ultrasonography (US) of the common extensor tendon (CET) of the elbow is often part of the assessment of patients with lateral epicondylitis. This US assessment is currently based on general tendinopathy references and not well-defined US entities.
Purpose:
To describe CET thickness, color Doppler activity, and bony spurs on US in asymptomatic volunteers and to investigate the influence of sex, age, height, body mass index (BMI), weight, and elbow dominance on the measurements.
Study Design:
Cross-sectional study; Level of evidence, 3.
Methods:
Tendon thickness, color Doppler activity, and bony spurs of the CET were measured sonographically in 264 adults (50% women) aged 20 to 96 years. Two different tendon-thickness measuring techniques were applied, labeled the “plateau measure” and the “1-cm measure.” Color Doppler activity was based on a 0 to 4 rating scale (negative, grades 0 and 1; positive, grades 2-4). A bony spur was defined as a bony outgrowth (≥0.3 mm) arising at the insertional site of the CET.
Results:
With both tendon-thickness measuring techniques, the CET in the dominant elbow was thicker than that in the nondominant elbow, and male tendons were thicker than female tendons (all P ≤ .03). In regression analysis, tendon thickness correlated with weight, color Doppler activity, and arm dominance for both measuring techniques in multiple regression analysis. In addition, the plateau measure correlated with height and the presence of bony spurs. No correlations were observed regarding BMI, sex, or age. Positive color Doppler activity was found in 9% of examined elbows, with no difference between the sexes regarding dominant versus nondominant elbows (all P ≥.20). Bony spurs were found to increase with age, from 23% for people in their 20s to 74% in people older than 70 years. Bony spurs were more common in the dominant elbow (P ≤ .01). Women had a higher prevalence of bony spurs than men, but only in the dominant elbow (P = .03).
Conclusion:
This study presents the US characteristics and normal values of the CET. In 264 asymptomatic participants, the CET was found to be thicker in men and in the dominant elbow. No difference in tendon thickness could be demonstrated with regard to different age groups. Color Doppler activity was found to be positive in nearly 1 of 10 asymptomatic subjects. Bony spurs were a common finding; they increased in prevalence with every decade in age and were considered part of the aging process. Normal variations in CET morphologic characteristics should therefore be considered when implementing US in trials and clinical practice.
Keywords: ultrasonography, tendinopathy, tennis elbow, lateral epicondylitis, common extensor tendon, healthy controls, tendon thickness, color Doppler activity, bony spurs
Lateral epicondylitis (LE), also known as tennis elbow, is a common musculotendinous disorder.12,39 The diagnosis of LE is defined as the presence of pain on the lateral side of the elbow and pain at the lateral epicondyle on direct palpation of the common extensor tendon (CET) and during resisted dorsiflexion of the wrist.28,31 As a supplement to the clinical investigation, ultrasonography (US) is often included to support the diagnosis and evaluate other potential causes of lateral elbow pain such as nerve entrapment (radial tunnel syndrome) and radiohumeral joint pathology.18
US plays a central role in sports medicine.9,11,13 General US findings in tendinopathy are described as an increase in tendon thickness, presence of color Doppler activity, irregular fibrillar appearance, calcifications, and hypoechoic areas.1,17,24,40 Several studies have adopted some of these US features in the assessment of patients with LE.4,5,22,23,25,29 However, the described tendinopathic changes have not been compared with those present in the general population, and the standards lack a scientifically based consensus. The establishment of valid standards will help to avoid misinterpretation and distinguish between pathological and benign US findings. To establish such standards requires normative data based on the examination of a large and heterogeneous cohort.
The purpose of this study was to evaluate CET thickness, color Doppler activity, and bony spurs on US in 264 asymptomatic participants and to investigate the influence of sex, age, height, body mass index (BMI), weight, and elbow dominance on the measurements.
Methods
Study Design and Participants
This was a cross-sectional observational study with 264 asymptomatic participants. The inclusion criterion was a bilateral pain-free clinical examination of the lateral elbow, with no pain at the lateral epicondyle on direct palpation or during resisted dorsiflexion of the wrist. Exclusion criteria were age younger than 20 years and any history of current or previous lateral elbow pain.
The study was conducted in 2 Danish cities, Aalborg (population, 80,000) and Østervrå (population, 1500). The participants were recruited at 2 different locations: a supermarket and a private orthopedic clinic where mainly minor surgery and sports medicine were performed; both clinic patients and accompanying family were invited to participate. Invitation to participate, informed consent, and the subsequent US examination took place on the same day. Participants were selected to ensure an equal distribution of sex and age groups. The study was submitted to the local ethical committee of Central Denmark Region (file reference number 1-10-72-18-16). The study did not require approval from the committee system. All participants gave informed consent. The study was carried out in accordance with Danish law and the Declaration of Helsinki.
Sonographic Evaluation
Patients were examined in a sitting position with the elbow flexed to 90°, the wrist pronated, and the arm resting on a table (Figure 1A). The anatomic landmarks of the lateral epicondyle are shown in Figure 1B. Longitudinal images were used for the evaluation. All scans were performed by a single physician (T.P.K.), a rheumatologist and sports physician with more than 10 years of US experience in sports medicine. The equipment used was a LOGIQ S8 (GE Healthcare) with a 15-MHz (MLG-15) linear transducer. Cross Beam was applied, and dynamic range was set to 66 dB.
Figure 1.
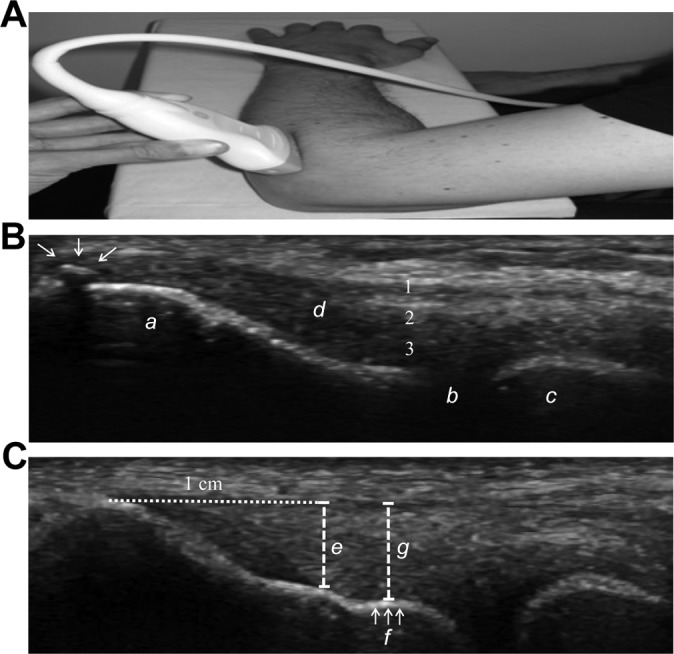
(A) Photograph of arm and longitudinal probe position. (B) Longitudinal ultrasonogram illustrating a bony spur (arrows) at the most proximal part of the insertion of the common extensor tendon (CET). Lateral epicondyle (a); radiohumeral joint (b); radial head (c); the CET (d). The numbers indicate the layers included in the measurement: (1) extensor digitorum communis, (2) extensor carpi radialis brevis, (3) joint capsule/radial ligament complex. (C) Longitudinal sonogram illustrating 2 different methods for measuring CET thickness. Dashed line (e) indicates tendon thickness measured 1 cm from the top of the lateral epicondyle (“1-cm measure”). Dashed line (g) indicates tendon thickness measured at the plateau (f) (“plateau measure”).
Tendon Thickness
Two different ways of measuring the thickness of the CET were applied (Figure 1C). Method 1, labeled “plateau measure,” measured tendon thickness at an anatomic landmark at the bony surface of the lateral epicondyle, which we refer to as “the plateau.” The plateau is a flat aspect of the capitulum of the lateral epicondyle located between the insertion of the tendon and the humeroradial joint. Tendon thickness was measured from the plateau to the tendon surface perpendicular to the length of the tendon. Method 2, labeled “1-cm measure,” measured tendon thickness 1 cm from the proximal part of the insertion of the CET (on top of the lateral epicondyle), perpendicular to the length of the tendon.19 The deepest structures, close to the bone, consist of ligamentous tissue and the lateral collateral ligament complex/joint capsule. Therefore, the 2 included measurements covered not only the CET but also to a varying degree the underlying ligamentous structures (Figure 1B).14 To what extent the ligamentous structures contribute to the overall thickness measurement depends on the location at the lateral epicondyle where the US is performed.27 At the anterior portion of the extensor carpi radialis brevis origin, the joint capsule is very thin, whereas at the posterior portion of the extensor carpi radialis brevis, a more robust attachment is formed that includes the joint capsule, annular ligament, and supinator, all intermingled.6,14,27 In this study, the probe was aimed at the central portion/middle part of the CET in an anterior to posterior orientation.
Color Doppler Assessment
Doppler settings were the same for all patients, with a gain setting just below the noise level and pulse repetition frequency set to 1.0. The transducer was aligned with the long axis of the radius over the CET. The CET was examined with color Doppler US in the longitudinal plane by moving the transducer from side to side, locating the part with the most Doppler activity. Probe pressure was limited to a minimum, and a thick layer of gel was applied. The color Doppler activity is usually seen in an area limited proximally by the tip of the lateral epicondyle and distally by the humeroradial joint space. The superficial border was the most superficial fibers of the CET, and the profound border was the bone. Color Doppler activity was graded on a scale from 0 to 4 (Figure 2).
Figure 2.
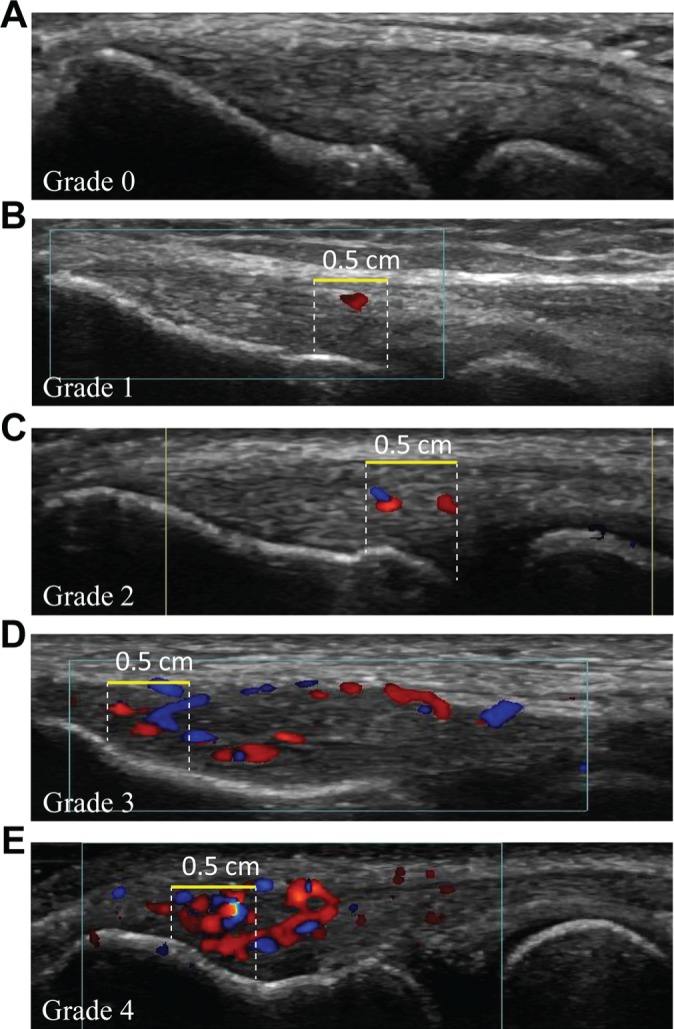
(A-E) Longitudinal ultrasonogram of the common extensor tendon (CET) illustrating grading of color Doppler activity from grade 0 to 4. The grading was performed in the region of interest (ROI), defined as a 0.5-cm longitudinal part of the tendon with maximum color Doppler activity. A horizontal yellow line measuring 0.5 cm marks the superficial border of the ROI, white dashed lines mark the proximal and distal borders, and the bone marks the deep border. (A) Grade 0: no activity. (B) Grade 1: single vessel in the ROI. (C) Grade 2: Doppler activity in less than 25% of the ROI. (D) Grade 3: Doppler activity in 25% to 50% of the ROI. (E) Grade 4: Doppler activity in more than 50% of the ROI.
The grading was estimated in a 0.5-cm longitudinal part of the tendon with the maximal Doppler activity (the region of interest [ROI]). Grading was as follows: grade 0, no activity; grade 1, single vessel in the ROI; grade 2, Doppler activity in less than 25% of the ROI; grade 3, Doppler activity in 25% to 50% of the ROI; and grade 4, Doppler activity in more than 50% of the ROI.19,20 The 5-point ranking scale was dichotomized to being either Doppler positive or Doppler negative, as we expected few participants with abnormal Doppler activity in the general population. Doppler activity grades 0 and 1 were regarded as Doppler negative, whereas grades 2 to 4 were regarded as Doppler positive.
Bony Spurs
A bony spur (entesophyte) was defined as a bony outgrowth arising at the insertional site of the CET (Figure 1B). The presence or absence of a bony spur was recorded. Any bony irregularity of less than 0.3 mm was not included.19
The US outcomes used in this trial have previously been applied to a randomized controlled trial and assessed in a reliability and agreement study.19,20 Good to excellent reliability was obtained for all measures. Depending on the measurement techniques used and whether an assisting template showing where to measure was used, tendon-thickness reliability ranged from 0.76 to 0.81 in the intraobserver study. In the interobserver study, reliability ranged from 0.45 to 0.65. The smallest detectable difference in tendon-thickness measurement ranged from 0.39 to 0.57 mm in the intraobserver study and 0.65 to 1.14 mm in the interobserver study.19 Color Doppler activity and bony spurs were only evaluated as an interobserver study and reliability was 0.93 and 1.0, respectively.19
Outcomes
Demographic information included, age, sex, BMI, and arm dominance. Color Doppler activity, bony spurs, and tendon thickness were assessed by US.
Statistical Analysis
Data are reported as mean with standard deviation. Comparisons of continuous variables between unpaired groups were made with a 2-sample t test, and the paired-samples t test was used for paired groups. Comparisons of categorical data between unpaired groups were made with Pearson χ2 test, and the McNemar test was used for paired groups. Possible predictors of tendon thickness were initially tested in univariate linear regression analyses. Afterward, multiple linear regression analyses were performed with backward selection, using the criterion of P ≥ .05 for removal from the model. The initial and final models are reported. Pearson correlation was performed to determine the relationship between the US measurements of tendon thickness by the plateau measure and 1-cm measure. Stata software version 13.1 was used for analysis. A P value less than .05 was considered to indicate statistical significance.
Results
A total of 264 participants (134 males, 134 females) with no current or previous history of LE were enrolled in the study. Only 5 invited persons refused to participate. US examination of the CET was performed on both elbows, assessing tendon thickness, color Doppler activity, and bony spurs.
The anthropometric characteristics for the men and women are listed in Table 1. The subjects varied in age (20-96 years) and adiposity (BMI, 16.4-52.1 kg/m2). All patients were White. The men were taller and heavier (both P <.001), but had similar BMI in comparison with the women (P = .6).
TABLE 1.
Demographic Informationa
| Characteristic | All Subjects | Women | Men |
|---|---|---|---|
| Number | 264 | 132 | 132 |
| Age, y, mean (SD) | 50.0 (18.7) | 50.3 (19.0) | 49.6 (18.4) |
| BMI, kg/m2, mean (SD) | 26.1 (4.5) | 25.9 (4.8) | 26.2 (4.2) |
| Body weight, kg, mean (SD) | 78.4 (15.4) | 71.4 (13.8) | 85.4 (13.6) |
| Height, cm, mean (SD) | 173.2 (10.1) | 165.9 (6.3) | 180.5 (7.6) |
| Dominant right hand, n (%) | 236 (89) | 123 (93.2) | 113 (86) |
aBMI, body mass index.
Tendon Thickness
Mean tendon thicknesses for men and women are listed in Table 2. For all measurements, the male tendon was significantly thicker than the female tendon and the dominant tendon was significantly thicker than the nondominant. With the plateau measure technique, the male tendon was 0.35 mm (6.9%) thicker than the female tendon in the dominant elbow (P < .001), and in the nondominant elbow the male tendon was 0.39 mm (7.9%) thicker than the female tendon (P < .001). With the 1-cm measure, the male tendon was found to be 0.18 mm (4.0%) thicker than the female tendon in the dominant elbow (P = .03) and 0.21 mm (4.8%) thicker in the nondominant elbow (P = .01).
TABLE 2.
Common Extensor Tendon Values for Tendon Thickness, Color Doppler Activity, and Bony Spursa
| Characteristic | All subjects (N = 264) | Women (n = 132) | Men (n = 132) |
|---|---|---|---|
| Tendon thickness, mm, mean (SD) | |||
| Plateau measure, dominant arm | 4.87 (0.78) | 4.70 (0.64) | 5.05 (0.86) |
| Plateau measure, nondominant arm | 4.72 (0.76) | 4.53 (0.63) | 4.92 (0.83) |
| 1-cm measure, dominant arm | 4.41 (0.69) | 4.32 (0.60) | 4.50 (0.76) |
| 1-cm measure, nondominant arm | 4.24 (0.66) | 4.13 (0.55) | 4.34 (0.74) |
| Color Doppler activity dominant arm, yes, n (%) | 24 (9) | 9 (7) | 15 (11) |
| Color Doppler activity nondominant arm, yes, n (%) | 24 (9) | 11 (8) | 13 (10) |
| Bony spur dominant arm, yes, n (%) | 132 (50) | 75 (57) | 57 (43) |
| Bony spur nondominant arm, yes, n (%) | 108 (41) | 59 (45) | 49 (37) |
aA 95% prediction interval for the tendon thickness can be calculated as follows: mean ± 1.96 × SD.
Table 3 shows the results of the regression analyses. With the exception of age, all variables correlated with tendon thickness in univariate analyses. In the final regression analyses tendon thickness correlated with weight, color Doppler, and arm dominance in both measurement methods. The plateau measure correlated in addition with bony spurs and height. No correlation was observed between tendon thickness and BMI, sex, or age.
TABLE 3.
Regression Analyses for Predictors of Tendon Thickness
| Tendon Thickness “Plateau Measure” | Tendon Thickness “1-cm Measure” | |||||
|---|---|---|---|---|---|---|
| Variable | r | Confidence Interval | P Value | r | Confidence Interval | P Value |
| Univariate analyses | ||||||
| Age | 0.003 | −0.0006 to 0.006 | .11 | 0.0003 | −0.003 to 0.003 | .85 |
| Sex | −0.37 | −0.50 to −0.24 | <.001 | −0.19 | −0.31 to -0.08 | .001 |
| Height | 1.81 | 1.18 to 2.45 | <.001 | 0.94 | 0.37 to 1.51 | .001 |
| Weight | 0.01 | 0.009 to 0.02 | <.001 | 0.009 | 0.005 to 0.01 | <.001 |
| BMI | 0.02 | 0.006 to 0.04 | .006 | 0.02 | 0.007 to 0.03 | .003 |
| Doppler positive | 0.62 | 0.40 to 0.85 | <.001 | 0.42 | 0.22 to 0.62 | <.001 |
| Bony spur | 0.27 | 0.14 to 0.40 | <.001 | 0.16 | 0.04 to 0.27 | .007 |
| Dominant arm | 0.15 | 0.02 to 0.28 | .03 | 0.18 | 0.06 to 0.29 | .003 |
| Initial models of multiple logistic regression analyses with backward selection | ||||||
| Constant | 2.09 | 0.26 to 3.92 | .03 | 3.49 | 1.81 to 5.17 | <.001 |
| Age | 0.002 | −0.002 to 0.006 | .26 | −0.001 | −0.004 to 0.003 | .60 |
| Sex | −0.13 | −0.31 to 0.06 | .18 | −0.08 | −0.25 to 0.08 | .32 |
| Height | 1.11 | 0.08 to 2.15 | .04 | 0.13 | −0.81 to 1.08 | .78 |
| Weight | 0.007 | 0.002 to 0.01 | .008 | 0.007 | 0.002 to 0.01 | .004 |
| Doppler positive | 0.52 | 0.30 to 0.73 | <.001 | 0.36 | 0.16 to 0.56 | <.001 |
| Bony spur | 0.25 | 0.12 to 0.38 | <.001 | 0.14 | 0.02 to 0.26 | .03 |
| Dominant arm | 0.13 | 0.004 to 0.25 | .043 | 0.16 | 0.05 to 0.28 | .004 |
| Final models of multiple logistic regression analyses with backward selection | ||||||
| Constant | 1.54 | 0.40 to 2.68 | .008 | 3.55 | 3.25 to 3.84 | <.001 |
| Weight | 0.007 | 0.003 to 0.01 | .003 | 0.008 | 0.005 to 0.01 | <.001 |
| Doppler positive | 0.52 | 0.30 to 0.73 | <.001 | 0.40 | 0.21 to 0.59 | <.001 |
| Dominant arm | 0.12 | 0.002 to 0.25 | .047 | 0.18 | 0.07 to 0.29 | .002 |
| Bony spur | 0.28 | 0.15 to 0.41 | <.001 | — | — | — |
| Height | 1.41 | 0.67 to 2.16 | <.001 | — | — | — |
aVariables included in the multiple regression model were age, sex, height, weight, BMI, Doppler positive, bony spur, and dominant arm. In the final model, bony spur and height were only included for the plateau measure. BMI, body mass index.
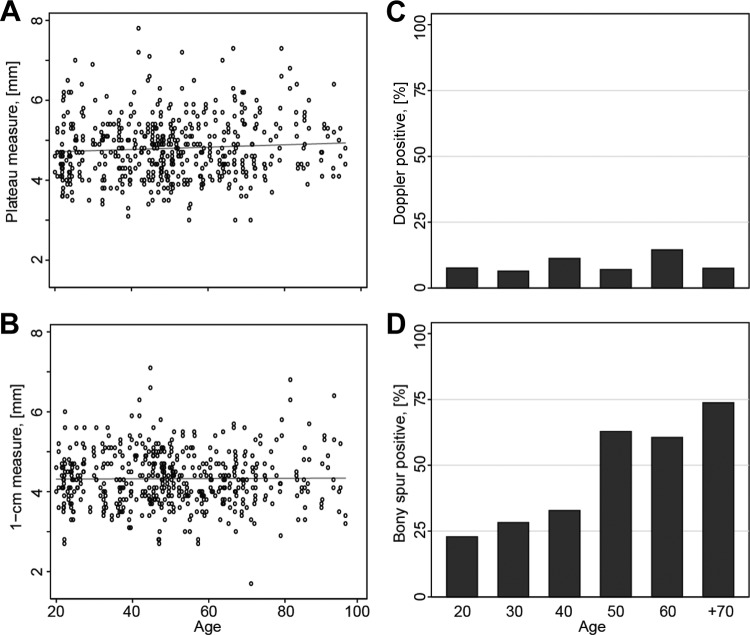
Figures 3A and 3B show the absence of a correlation between age and tendon thickness for both the plateau and 1-cm measuring techniques. Figures 4A and 4B show the distribution of tendon thickness for men and women for both techniques. For all measurements, the dominant tendon was significantly thicker than the nondominant tendon. With the plateau measure, the dominant tendon was found to be 0.17 mm (3.6%) thicker than the nondominant tendon in women (P < .001), 0.13 mm (2.6%) in men (P = .004), and 0.15 mm (3.1%) in all subjects together (P < .001). With the 1-cm measure, the dominant tendon was found to be 0.19 mm (4.4%) thicker than the nondominant tendon in women (P < .001), 0.17 mm (3.6%) in men (P < .001), and 0.18 mm (3.9%) in all subjects (P < .001).
Figure 3.
(A) Association between tendon thickness and age (plateau measure). (B) Association between tendon thickness and age (1-cm measure). (C) The frequency of positive color Doppler activity for each decade in age in both men and women, dominant and nondominant elbows combined. (D) The frequency of bony spurs for each decade in age for both men and women, dominant and nondominant arms combined.
Figure 4.
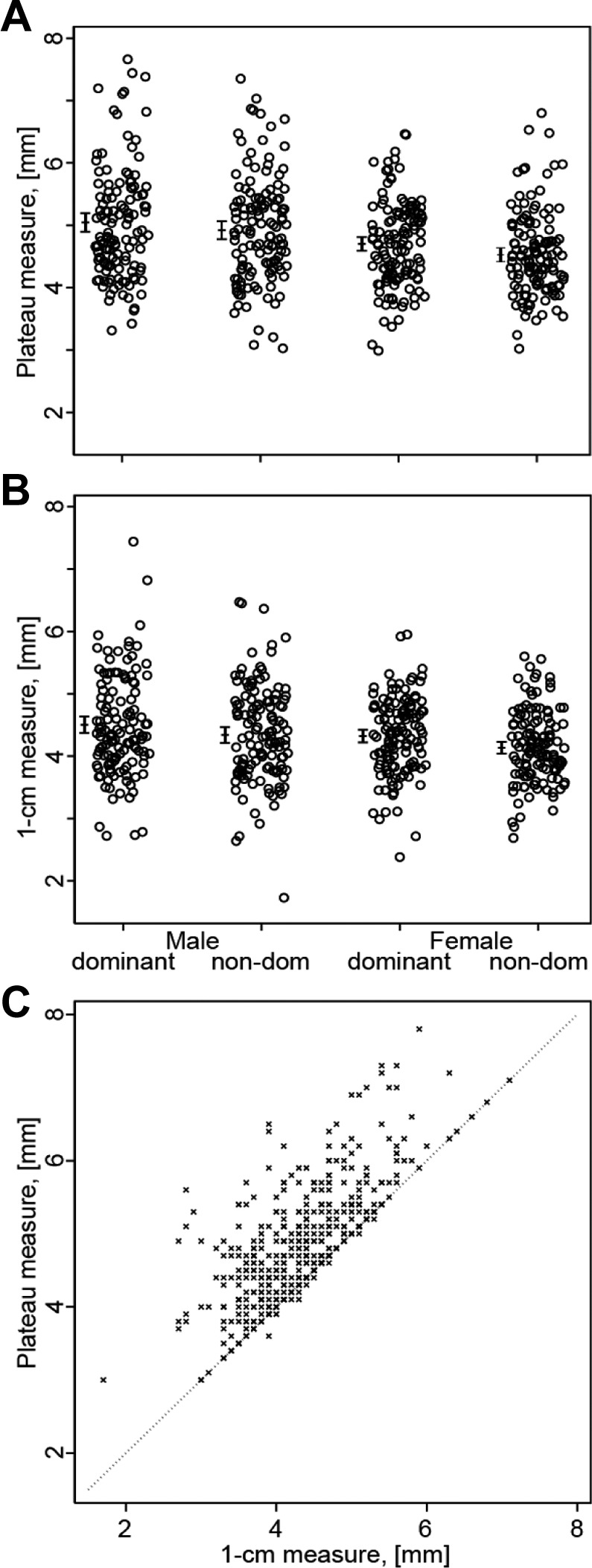
(A) The distribution of tendon thickness of the common extensor tendon (CET) between men and women in the dominant elbow based on the plateau measure. (B) The distribution of tendon thickness of the CET between men and women in the dominant elbow based on the 1-cm measure. (C) Positive association between the 1-cm measure (x-axis) and plateau measure (y-axis). Combined analyses of tendon thickness measures of both the dominant and nondominant elbows (N = 528), r = 0.73, P < .001.
There was a positive correlation between the plateau and the 1-cm measure for both the dominant and nondominant elbows, r = 0.70 and r = 0.75, respectively, P < .001. Figure 4C shows the correlation between the plateau measure and 1-cm measure, with the dominant and nondominant elbows combined in the analysis, r = 0.73, P < .001.
Color Doppler Activity
Table 2 lists the overall number and percentage of participants with positive color Doppler activity. There was no significant difference between men and women in either the dominant elbow, 11.4% versus 6.8% (P = .67), or in the nondominant elbow, 9.8% versus 8.3% (P = .20). Nor was there a difference between the dominant and nondominant elbow for men (11.4% vs 9.8%; P = .62), for women (6.8% vs 8.3%; P = .62), or for all subjects together (9.1% vs 9.1%; P = 1). Figure 3C shows the even distribution of positive color Doppler activity in the different age groups. The distribution of color Doppler activity in the 528 examined elbows according to the 0-to-4 grading scale was as follows: grade 0 was observed in 458 elbows (86.7%), grade 1 in 22 (4.2%), grade 2 in 25 (4.7%), grade 3 in 20 (3.8%), and grade 4 in 3 (0.6%).
Bony Spurs
Table 2 lists the number and percentage of participants with a bony spur. It is a common finding in both sexes, and its frequency increases with age (Figure 3D). There was a higher prevalence of bony spur in women (56.8%) than in men (43.2%), P = .03, in the dominant elbow. But in the nondominant elbow, the difference between women and men was not statistically significant (44.7% vs 37.1%; P = .20). Bony spurs were more common in the dominant elbow than in the nondominant in women, 56.8% versus 44.7% (P < .001), men, 43.2% versus 37.1% (P = .01), and all subjects, 50.0% versus 40.9% (P < .001).
Discussion
In this study, we investigated the US appearance of the CET in a healthy population without any LE history. We describe factors associated with tendon thickness and the prevalence of Doppler activity and bony spurs. The US outcomes used to evaluate LE patients are generally not well documented. They are often based on either clinical experience or inferred from tendinopathies in other areas of the body. This current study differs from other studies in the same field by being the first to apply US methods that have been scientifically investigated prior to including them as outcome measures. To be able to discuss what is a normal tendon appearance and what is abnormal requires standardized methods. This is a limitation in the majority of studies on US and tendon research in general. To compare studies and reproduce results becomes challenging when each group of US researchers make their own set of US definitions, which has been the case in the early years of US research. Our study stands out by evenly addressing 3 different US modalities that have been previously defined and investigated scientifically.19 Thus the results from this study can be applied to future research on the topic because of the documented reproducibility of the US methods. Furthermore, this study included a large participant number covering a broad age spectrum equally distributed between men and women. This allowed for investigating US changes related to age or those occurring with a low frequency.
Two studies, by Ustuner et al38 and Jaén-Díaz et al,15 have previously described US findings in the lateral elbow in asymptomatic individuals. Ustuner et al38 looked at a cohort of 100 participants, all health personnel of which 80% were women. They primarily addressed tendon thickness but also reported tears, effusion, neovascularization, spurs, bone irregularities, enthesophytes, and tendon calcifications. The examined cohort was probably too homogenous to represent the general population, contrary to the participants in our study. Jaén-Díaz et al15 included 240 patients evenly divided between men and women from an urban health district. The study design has many similarities to our study. Like Ustuner et al,38 the primary focus area was tendon thickness, but color Doppler, spurs, intratendinous calcifications, and abnormalities in the epicondylar bone cortex were also reported. In all, 15% of the included participants had a history of LE. The authors argued that the cohort was unlikely to represent the general population. As was the case in the study by Ustuner et al,38 the included US methods had not been scientifically investigated for the examination of the CET.
Tendon Thickness
In both the plateau and the 1-cm methods in our study, men were found to have a 4.0% to 7.9% thicker CET compared with women. However, sex was not a significant predictor of tendon thickness in the multivariate regression analyses, while dominant arm, weight, color Doppler activity, height, and bony spurs were (height and bony spurs were only predictors for the plateau measure). Our results indicate that when it comes to tendon tissue, sex is not a predictor for tendon thickness as is the case, for example, in skeletal muscle mass.16 In the current study, the men were 14 kg heavier and 14.6 cm higher than the women, and these differences in weight and height could explain the observed CET thickness differences between men and women. The reason that height and weight appear to be predictors for tendon thickness is thought to be the positive association between muscle mass/longer bones and a proportionally stronger and thus thicker tendon/enthesis. BMI, on the other hand, was not found to be a predictor of tendon thickness. This is because of the nature of the mathematical BMI formula, where 2 people with different muscle mass can have the same BMI.
The CET of the dominant elbow was consistently thicker compared with the nondominant side for example, 0.12 mm thicker with the plateau measure and 0.18 mm with the 1-cm measure. This is most likely explained by a higher loading on the dominant arm that results in a relative tendon hypertrophy compared with the nondominant arm. There is a large variation in tendon thickness for both measurements, and this variation could hinder the generation of a cutoff value differentiating a healthy tendon from a tendinopathic tendon. The contralateral elbow would then serve as a better comparator than the general population. In this regard, the small dominant-versus-nondominant elbow difference would probably be negligible.
A priori, we expected older age to be associated with a decrease in tendon thickness, similar to the decrease seen in skeletal muscle. Our data were not able to support this hypothesis because we found no age-related differences in tendon thickness in either the linear regression analyses or in the direct comparison of age groups (Figure 3, A and B). That is not to say that the CET and the underlying ligamentous structures do not change over time, but in our model, we were not able verify thickness changes.
In the literature, a few studies have looked at tendon thickness in the general population. In accordance with our findings, Ustuner et al38 found the CET to be thicker on the dominant side. In contrast to our study, they found tendon thickness to correlate moderately with age and BMI. Jaén-Díaz et al15 found tendon thickness to be greater in the dominant arm and in men, which is in accordance with our study. In their linear regression analysis, they made separate analyses for men and women and found a weak association with age and a modest association with weight. Jaén-Diaz et al15 and Ustuner et al38 used a method for measuring tendon thickness similar to the plateau measure used in our study. As an example of the similarities between the values for tendon thickness in the 2 studies, Jaén-Díaz et al15 found tendon thickness in the dominant elbow in men to be 5.09 mm, compared with 5.05 mm (plateau measure) in our study.
Two Different Methods
We applied 2 different methods for measuring CET thickness. These methods have previously been assessed in a methodological study on asymptomatic and LE patients regarding reliability and agreement, with an excellent intraclass correlation and moderate to good interclass correlation.19 The plateau measure and 1-cm measure correlate nicely with a positive correlation coefficient of 0.73 (Figure 4C), with the plateau measure measuring a thicker part of the tendon. The 2 ways of measuring tendon thickness differ in one important way: The plateau measure benefits from a fixed anatomic landmark, and the part of the CET being measured is unaffected by the length of the lateral epicondyle. The 1-cm measure assesses the CET at a distance of 1 cm from the top of the lateral epicondyle. In an epicondyle with a short bone length, the 1-cm measure calculation is closer to the landmark of the plateau measure, which is also the part of the epicondyle with the thickest CET (Figure 1B). In an epicondyle with a long bone length, the opposite is the case, and the 1-cm measure is at a thinner aspect of the CET. This could explain why height was a predictor for tendon thickness in the plateau measure but not in the 1-cm measure. Which of the methods would be most clinically relevant in evaluating LE patients cannot be answered from this study. Future trials need to compare the data from this study to data obtained in LE patients in order to verify if LE tendons change in thickness as part of the tendinopathic picture. Jaén-Díaz et al15 compared tendon thickness in 204 asymptomatic volunteers to 36 participants with a history of epicondylar pain. They found a 5% to 10% difference, indicating that symptomatic tendons increase in thickness.
Color Doppler Activity
The 0-to-4 grading scale for color Doppler activity has been assessed for reliability and agreement, with excellent interclass correlation, and applied to a randomized controlled trial.19,20 The prevalence of Doppler positivity (grades 2-4) was 9%, with no difference between the dominant and non-dominant arms or between men and women. The prevalence was consistent through the age groups, as shown in Figure 3C. The presence of color Doppler positivity was associated with an increase in tendon thickness of 0.40 to 0.52 mm. It may be a normal finding that tendinous tissue such as the CET displays increased blood flow from time to time, for example, after a period with increased tendon loading. Alternatively, the changes could represent actual tendinopathy but without symptoms (a subclinical tendinopathy—the iceberg theory).10 In a study by Boesen et al3 on Achilles and patellar tendons, a 0-to-5 grading scale was used to asses color Doppler activity in asymptomatic elite badminton players. Similar to our study, grades 0 and 1 were considered normal and grades 2 to 5 pathological. A high prevalence (43%) of grades 2 to 5 blood flow was observed in asymptomatic Achilles and patellar tendons. The authors argued that this could be part of a physiological adaptive response to loading.3 Compared with our study, it is important to remember that the study by Boesen et al3 was based on participants with a much higher tendon loading than the general population, and that the tendon structures investigated were different from the CET and therefore not directly comparable. In LE studies, Doppler activity is often regarded as a US characteristic of an ongoing tendinopathy.4,8,42 The justification of color Doppler as a tool in the diagnostic work-up and follow-up of LE patients cannot be further addressed in this article. Nevertheless, based on the prevalence of color Doppler activity in this study, the interpretation of Doppler activity should be approached with caution. Ustuner et al38 found no Doppler (power Doppler) activity in the 200 elbows examined, in contrast to the 9% in our study. A possible explanation could be the difference in US equipment used. We used high-end equipment with a high-end probe that is more likely to detect color Doppler activity.38 Jaén-Díaz et al15 found only 2 participants with positive color Doppler activity. Again, this is a very small number compared with our study and is most likely due to equipment quality with limited Doppler sensitivity.15
Bony Spurs
The reliability and agreement of bony spur evaluation used in this study has previously been assessed with a perfect interclass correlation.19 A bony spur formation arises at an enthesis (an enthesophyte) and extends in the direction of pull of a ligament or tendon.34 Only a spur of 0.3 mm or more was included in this study. In our study, we found bony spurs to be a very common finding. The prevalence increased with age, from 23% for patients in their 20s to 74% in those older than 70 years. As seen in Figure 3D, the biggest changes in prevalence appear to take place from the 4th to the 5th decade. Our findings indicate that spur formation to a large extent is age related and part of the aging process for a proportion of the population (bone formers).34 However, spurs can represent an underlying pathological picture, apart from being degenerative in nature or without a clear underlying cause.2,32,34 Conditions associated with bony spur formation include spondyloarthritis, psoriatic arthritis, and various endocrine disorders such as diabetes mellitus, local trauma, and calcium pyrophosphate deposition disease.26,32,35 In many studies investigating LE with the use of US, the presence of spurs is described as a sonographic characteristic of tendinopathy.8,22,29,30 Bony spurs might occur with a higher prevalence or size in patients with LE. However, based on the findings in our study, the clinical value of describing a bony spur is limited because it is a very common phenomenon. The presence of a bony spur is unlikely to either rule in or rule out the possibility of the patient having LE. A bony spur was found to be a predictor of increased tendon thickness with the plateau measure and was borderline significant with the 1-cm measure (P = .054). This association can be explained by considering the enthesis organ as one entity undergoing degenerative processes with resulting structural changes, for example, bony spurring and increase in tendon thickness. Another theory could be that the spur extends the epicondyle and thereby “lifts” the insertion of the most superficial part of the CET, resulting in an increased thickness measure.
The prevalence of bony spurs is high in both the dominant as well as the nondominant arm. Regarding sex-based differences, we found spurs to be more common in the dominant elbow in women (56.8%) than in men (43.2%), but there was no significant difference with regard to the nondominant elbow, 44.7% versus 37.1%. The reason for this difference is unclear. In both men and women, spurs were found at a higher prevalence in the dominant arm compared with the nondominant arm. This association suggests that loading and bony spur formation are related. Ustuner et al38 presented no clear definition of the bony spur assessment. The study was not designed to demonstrate an age relation, but they stated that spurs are more common in the oldest 50% of the group. Jaén-Díaz et al15 defined a bony spur in a manner similar to our study although they did not have a lower limit for the size of the spur. In the different age groups, Jaén-Díaz et al15 observed bony spurs to increase with age and found prevalences very similar to our study. Most previous studies on bony spurs have been based on either direct examination of skeletal bones or on radiographs. In our literature search, we found no studies of this type with regard to LE, whereas studies on calcaneal spurs are common. Similar to our results, calcaneal spurs increase with age and tend to be seen more often in women; however, that has not been consistently reported in all studies.26,33,34,37
Limitations
We did not investigate all available US outcomes, as we only included outcomes that had been investigated methodologically.19 Additional areas of importance may be cross-sectional area of CTE, focal hypoechoic changes, partial ruptures, intratendon calcifications, bony erosion, the lateral ulnar collateral ligament, and elastography.5,7,21,36,41 The participants in this study stated that they had no history of LE. However, in a large cohort some participants may have forgotten a previous episode of LE. Many of the elderly in this study came from a rural community with a history of farm work, which could make this age group hard to compare with a general population with a history of less physically demanding work. All subjects in the study were White, thus it is possible that race may have an effect on the US measurements. A limitation in this study is the lack of subgroup analyses investigating the role of work- or sports-related activities. Future studies should look at the influence of sports/work on tendon measurements.
Conclusion
We examined the CET with US in 264 asymptomatic participants and found no age-related differences in tendon thickness. Color Doppler activity was seen, with an average prevalence of 9% but without any difference with regard to sex, arm dominance, or age. Bony spurs were very common and increased with age, for example, 3 out of 4 subjects older than 70 years had bony spurs. Normal variations of CET morphologic characteristics should therefore be considered when implementing US in trials and clinical practice.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: T.P.K. received a 6-month grant from the Danish Rheumatism Association and a 3-month grant from the Health Research Fund of Central Demark Region.
Ethical approval for this study was waived by the local ethical committee of Central Denmark Region.
References
- 1. Allen GM, Wilson DJ. Ultrasound in sports medicine—a critical evaluation. Eur J Radiol. 2007;62:79–85. [DOI] [PubMed] [Google Scholar]
- 2. Benjamin M, Rufai A, Ralphs JR. The mechanism of formation of bony spurs (enthesophytes) in the achilles tendon. Arthritis Rheum. 2000;43:576–583. [DOI] [PubMed] [Google Scholar]
- 3. Boesen AP, Boesen MI, Torp-Pedersen S, et al. Associations between abnormal ultrasound color Doppler measures and tendon pain symptoms in badminton players during a season: a prospective cohort study. Am J Sports Med. 2012;40:548–555. [DOI] [PubMed] [Google Scholar]
- 4. Clarke AW, Ahmad M, Curtis M, Connell DA. Lateral elbow tendinopathy: correlation of ultrasound findings with pain and functional disability. Am J Sports Med. 2010;38:1209–1214. [DOI] [PubMed] [Google Scholar]
- 5. Connell D, Burke F, Coombes P, et al. Sonographic examination of lateral epicondylitis. AJR Am J Roentgenol. 2001;176:777–782. [DOI] [PubMed] [Google Scholar]
- 6. De Maeseneer M, Brigido MK, Antic M, et al. Ultrasound of the elbow with emphasis on detailed assessment of ligaments, tendons, and nerves. Eur J Radiol. 2015;84:671–681. [DOI] [PubMed] [Google Scholar]
- 7. De Zordo T, Lill SR, Fink C, et al. Real-time sonoelastography of lateral epicondylitis: comparison of findings between patients and healthy volunteers. AJR Am J Roentgenol. 2009;193:180–185. [DOI] [PubMed] [Google Scholar]
- 8. duToit C, Stieler M, Saunders R, Bisset L, Vicenzino B. Diagnostic accuracy of power Doppler ultrasound in patients with chronic tennis elbow. Br J Sports Med. 2008;42:872–876. [DOI] [PubMed] [Google Scholar]
- 9. Fredberg U, Bolvig L. Significance of ultrasonographically detected asymptomatic tendinosis in the patellar and achilles tendons of elite soccer players: a longitudinal study. Am J Sports Med. 2002;30:488–491. [DOI] [PubMed] [Google Scholar]
- 10. Fredberg U, Bolvig L, Andersen NT. Prophylactic training in asymptomatic soccer players with ultrasonographic abnormalities in Achilles and patellar tendons: the Danish Super League Study. Am J Sports Med. 2008;36:451–460. [DOI] [PubMed] [Google Scholar]
- 11. Grassi W, Cervini C. Ultrasonography in rheumatology: an evolving technique. Ann Rheum Dis. 1998;57:268–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hamilton PG. The prevalence of humeral epicondylitis: a survey in general practice. J R Coll Gen Pract. 1986;36:464–465. [PMC free article] [PubMed] [Google Scholar]
- 13. Jacobson JA. Ultrasound in sports medicine. Radiol Clin North Am. 2002;40:363–386. [DOI] [PubMed] [Google Scholar]
- 14. Jacobson JA, Chiavaras MM, Lawton JM, Downie B, Yablon CM, Lawton J. Radial collateral ligament of the elbow: sonographic characterization with cadaveric dissection correlation and magnetic resonance arthrography. J Ultrasound Med. 2014;33:1041–1048. [DOI] [PubMed] [Google Scholar]
- 15. Jaén-Díaz JI, Cerezo-López E, López-de Castro F, et al. Sonographic findings for the common extensor tendon of the elbow in the general population. J Ultrasound Med. 2010;29:1717–1724. [DOI] [PubMed] [Google Scholar]
- 16. Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985). 2000;89:81–88. [DOI] [PubMed] [Google Scholar]
- 17. Joshua F, Edmonds J, Lassere M. Power Doppler ultrasound in musculoskeletal disease: a systematic review. Semin Arthritis Rheum. 2006;36:99–108. [DOI] [PubMed] [Google Scholar]
- 18. Konin GP, Nazarian LN, Walz DM. US of the elbow: indications, technique, normal anatomy, and pathologic conditions. Radiographics. 2013;33:E125–E147. [DOI] [PubMed] [Google Scholar]
- 19. Krogh TP, Fredberg U, Christensen R, Stengaard-Pedersen K, Ellingsen T. Ultrasonographic assessment of tendon thickness, doppler activity and bony spurs of the elbow in patients with lateral epicondylitis and healthy subjects: a reliability and agreement study. Ultraschall Med. 2013;34:468–474. [DOI] [PubMed] [Google Scholar]
- 20. Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41:625–635. [DOI] [PubMed] [Google Scholar]
- 21. Lee MH, Cha JG, Jin W, et al. Utility of sonographic measurement of the common tensor tendon in patients with lateral epicondylitis. AJR Am J Roentgenol. 2011;196:1363–1367. [DOI] [PubMed] [Google Scholar]
- 22. Levin D, Nazarian LN, Miller TT, et al. Lateral epicondylitis of the elbow: US findings. Radiology. 2005;237:230–234. [DOI] [PubMed] [Google Scholar]
- 23. Maffulli N, Regine R, Carrillo F, Capasso G, Minelli S. Tennis elbow: an ultrasonographic study in tennis players. Br J Sports Med. 1990;24:151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martinoli C, Bianchi S, Derchi LE. Tendon and nerve sonography. Radiol Clin North Am. 1999;37:691–711. [DOI] [PubMed] [Google Scholar]
- 25. Miller TT, Shapiro MA, Schultz E, Kalish PE. Comparison of sonography and MRI for diagnosing epicondylitis. J Clin Ultrasound. 2002;30:193–202. [DOI] [PubMed] [Google Scholar]
- 26. Moroney PJ, O’Neill BJ, Khan-Bhambro K, O’Flanagan SJ, Keogh P, Kenny PJ. The conundrum of calcaneal spurs: do they matter? Foot Ankle Spec. 2014;7:95–101. [DOI] [PubMed] [Google Scholar]
- 27. Nimura A, Fujishiro H, Wakabayashi Y, Imatani J, Sugaya H, Akita K. Joint capsule attachment to the extensor carpi radialis brevis origin: an anatomical study with possible implications regarding the etiology of lateral epicondylitis. J Hand Surg Am. 2014;39:219–225. [DOI] [PubMed] [Google Scholar]
- 28. Pienimaki T, Tarvainen T, Siira P, Malmivaara A, Vanharanta H. Associations between pain, grip strength, and manual tests in the treatment evaluation of chronic tennis elbow. Clin J Pain. 2002;18:164–170. [DOI] [PubMed] [Google Scholar]
- 29. Poltawski L, Jayaram V, Watson T. Measurement issues in the sonographic assessment of tennis elbow. J Clin Ultrasound. 2010;38:196–204. [DOI] [PubMed] [Google Scholar]
- 30. Radunovic G, Vlad V, Micu MC, et al. Ultrasound assessment of the elbow. Med Ultrason. 2012;14:141–146. [PubMed] [Google Scholar]
- 31. Regan W, Grondin P, Morrey BF. Lateral epicondylitis (tennis elbow) In: DeLee J, Drez D, Miller M, eds. DeLee and Drez’s Orthopaedic Sports Medicine. 3rd ed Amsterdam, Netherlands: Elsevier; 2009:1197–1204. [Google Scholar]
- 32. Resnick D, Niwayama G. Entheses and enthesopathy. Anatomical, pathological, and radiological correlation. Radiology. 1983;146:1–9. [DOI] [PubMed] [Google Scholar]
- 33. Riepert T, Drechsler T, Urban R, Schild H, Mattern R. The incidence, age dependence and sex distribution of the calcaneal spur. An analysis of its x-ray morphology in 1027 patients of the central European population. Rofo. 1995;162:502–505. [DOI] [PubMed] [Google Scholar]
- 34. Rogers J, Shepstone L, Dieppe P. Bone formers: osteophyte and enthesophyte formation are positively associated. Ann Rheum Dis. 1997;56:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shaibani A, Workman R, Rothschild BM. The significance of enthesopathy as a skeletal phenomenon. Clin Exp Rheumatol. 1993;11:399–403. [PubMed] [Google Scholar]
- 36. Stewart B, Harish S, Oomen G, Wainman B, Popowich T, Moro JK. Sonography of the lateral ulnar collateral ligament of the elbow: study of cadavers and healthy volunteers. AJR Am J Roentgenol. 2009;193:1615–1619. [DOI] [PubMed] [Google Scholar]
- 37. Toumi H, Davies R, Mazor M, et al. Changes in prevalence of calcaneal spurs in men & women: a random population from a trauma clinic. BMC Musculoskelet Disord. 2014;15:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ustuner E, Toprak U, Baskan B, Oztuna D. Sonographic examination of the common extensor tendon of the forearm at three different locations in the normal asymptomatic population. Surg Radiol Anat. 2013;35:547–552. [DOI] [PubMed] [Google Scholar]
- 39. Verhaar JA. Tennis elbow. Anatomical, epidemiological and therapeutic aspects. Int Orthop. 1994;18:263–267. [DOI] [PubMed] [Google Scholar]
- 40. Wakefield RJ, Balint PV, Szkudlarek M, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32:2485–2487. [PubMed] [Google Scholar]
- 41. Walz DM, Newman JS, Konin GP, Ross G. Epicondylitis: pathogenesis, imaging, and treatment. Radiographics. 2010;30:167–184. [DOI] [PubMed] [Google Scholar]
- 42. Zeisig E, Ohberg L, Alfredson H. Extensor origin vascularity related to pain in patients with tennis elbow. Knee Surg Sports Traumatol Arthrosc. 2006;14:659–663. [DOI] [PubMed] [Google Scholar]