Figure 4.
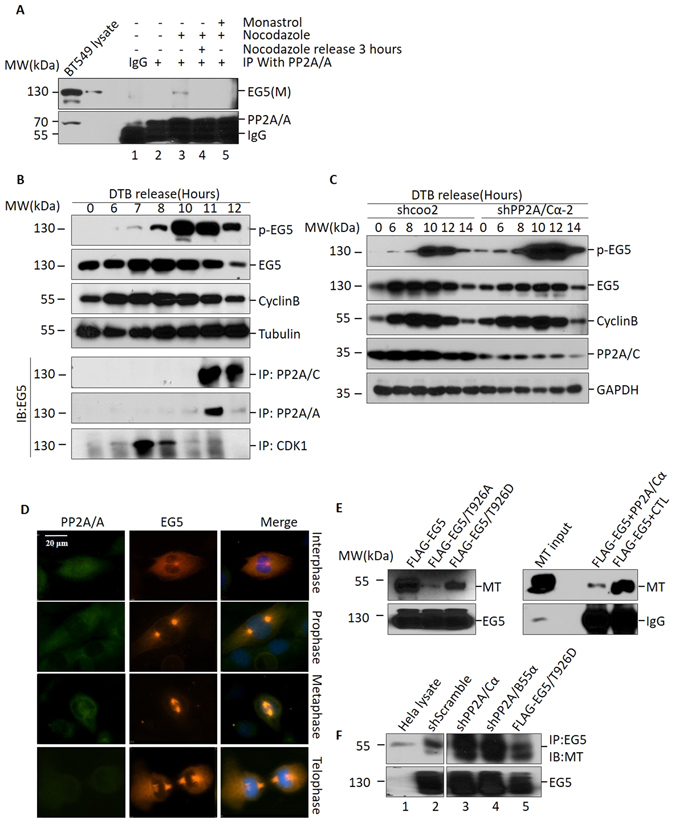
PP2A associates with EG5 localization during mitosis. (A) Co-immunoprecipitation of EG5 with PP2A/A after Nocodazole treatment. BT549 cells were treated with Nocodazole or Monastrol as indicated, then subjected to immunoprecipitation with anti-PP2A/A antibody followed by Western blot using anti-EG5 antibody. (B) HeLa cells were synchronized at G1/S with a double thymidine block (DTB), and were then released into fresh medium and harvested at indicated time points. Cell extracts were subjected to Western blot for evaluation of protein levels of phosphorylated EG5 (T926), total EG5 and cyclin B. Tubulin was used as loading control (upper panel). These cells were also subjected to co-immunoprecipitation with anti-PP2A/A, anti-PP2A/C and anti-CDK1 antibodies, and were then evaluated with anti-EG5 antibody. (C) HeLa cells infected with shPP2A/Cα-2 knockdown lenti-virus or control virus were synchronized with DTB and released into fresh medium. Whole cell lysate was harvested at indicated time points, then subjected to Western blot using anti-pEG5 (T926), anti-EG5, anti-cyclin B, anti-PP2A/C, or anti-tubulin antibodies. (D) Subcellular localization of PP2A/A and EG5 during mitosis in BT549 cells. Anti-EG5 and anti-PP2A/A antibodies were used to label endogenous EG5 and PP2A/A. DAPI was used to show chromatin status. Bar represents 10 μm. (E) Microtubule (MT) binding of EG5 and variants (left panel). MT purified from mouse brain was co-immunoprecipated with EG5 and its variants (T926A, T926D), then subjected to Western blot to evaluate the MT binding ability of EG5 and its variants. MT binding ability of EG5 decreased after PP2A/Cα treatment (right panel). (F) MT binding of endogenous EG5 in HeLa cells with decreased PP2A/Cα or PP2A/B55α, or overexpressed FLAG-EG5/T926D. For the original uncropped western blot data, see Supplementary information.
