Table 1. Optimization of the reaction conditions a .
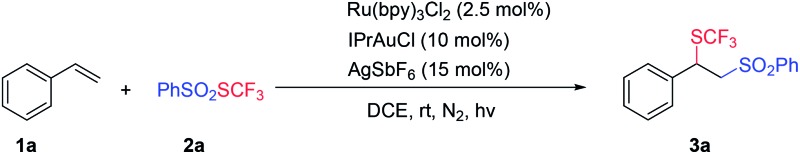
| ||
| Entry | Variation from the “standard” conditions | Yield b (%) |
| 1 | None | 94 (87) |
| 2 | AuCl instead of IPrAuCl | 0 |
| 3 | PPh3AuCl instead of IPrAuCl | 56 |
| 4 | IMesAuCl instead of IPrAuCl | 72 |
| 5 | PPh3AuNTf2 instead of IPrAuCl and AgSbF6 | 67 |
| 6 c | IPrAuSbF6 instead of IPrAuCl and AgSbF6 | 88 |
| 7 | CH3CN instead of DCE | 5 |
| 8 | CH3OH instead of DCE | 4 |
| 9 | Ru(phen)3Cl2 instead of Ru(bpy)3Cl2 | 67 |
| 10 | Ir[dF(CF3)ppy]2(dtbbpy)PF6 | 0 |
| 11 | fac-Ir(ppy)3 instead of Ru(bpy)3Cl2 | 0 |
| 12 | IPrAuCl (5 mol%), AgSbF6 (7.5 mol%), Ru(bpy)3Cl2 (1.2 mol%) | 64 |
aReaction conditions: a mixture of 1a (0.4 mmol), 2a (0.2 mmol), IPrAuCl (10 mol%), AgSbF6 (15 mol%), Ru(bpy)3Cl2 (2.5 mol%), in DCE (1 mL) was stirred at rt under irradiation with a 100 W blue LED at N2 atmosphere.
bDetermined by 19F NMR using (trifluoromethyl)benzene as the internal standard. The number in parentheses is the isolated yield. IPr = 1,3-bis(2,6-diisopropyl-phenyl)imidazol-2-ylidene, ppy = 2-phenylpyridine.
cRu(bpy)3(PF6)2 instead of Ru(bpy)3Cl2.
