Table 2. Substrate scope of alkene trifluoromethylthiosulfonylation reactions a .
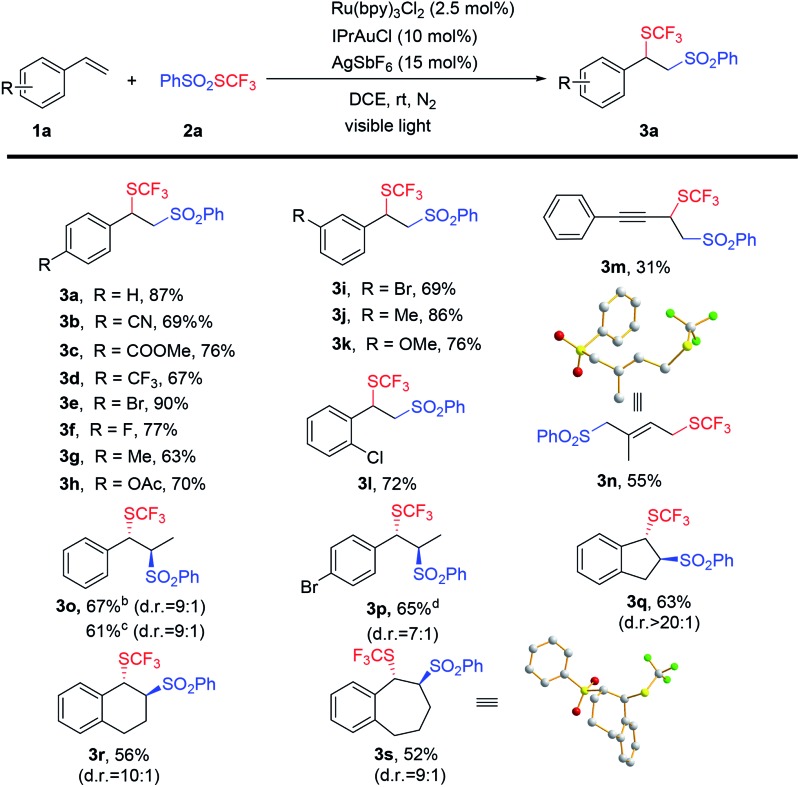
|
aStandard conditions were employed. Isolated yields were reported. Diastereoselectivities were determined by GC-mass.
bFrom (E)-alkene.
cFrom (Z)-alkene.
dFrom (Z)-alkene : (E)-alkene = 7 : 3.
