Figure 7.
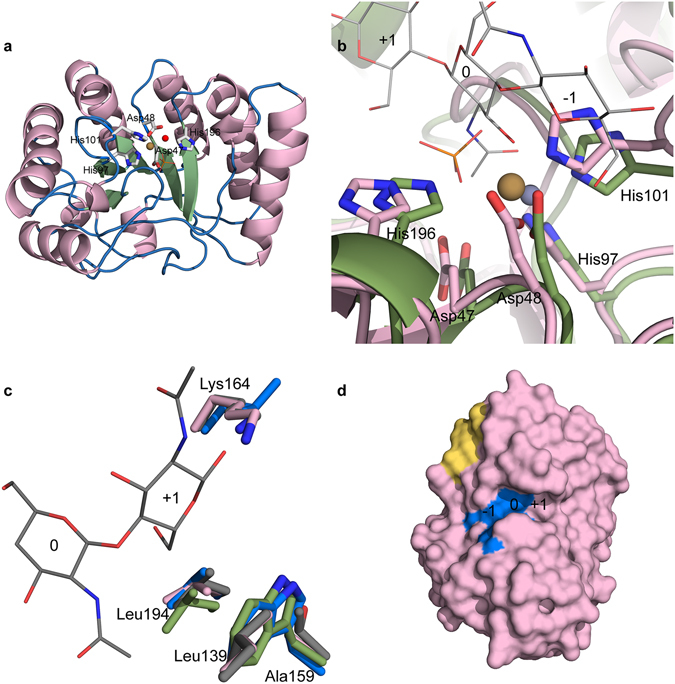
Crystal structure of AnCDA. (a) Structure of AnCDA coloured by secondary structure; green = strand, pink = helix and blue = loop/coil. The side chains of the metal binding residues, the catalytic acid and the catalytic base are labelled and shown as sticks, the phosphate ion is shown as orange lines, and the metal ion is shown as a brown sphere. The water ion participating in the octahedral arrangement of the metal is shown as a red sphere. (b) Structural superposition of AnCDA (pink) and VcCDA (green; PDB id: 4OUI)18 with a (GlnNAc)3 (grey lines) in the active site. The side chains of the metal binding His-His-Asp triad and the catalytic acid and base are shown as sticks, with numbering according to AnCDA. The phosphate ion in the AnCDA structure is shown as orange sticks. Subsites are labelled −1, 0, and +1. The brown sphere is the Co2+ ion bound in AnCDA, while the dark grey sphere is the Zn2+ ion bound in VcCDA. The structural superposition was generated using the PyMod 2.051 plugin in PyMol. (c) Structural differences around subsite +1 in AnCDA (pink), VcCDA (green), SpPgdA (blue; PDB id: 2C1G)15, and ClCDA (grey; PDB id: 2IW0)9, showing the sidechains (in sticks) of the residues forming the hydrophobic pocket and the lysine special for AnCDA and ClCDA (see text). The ligand is shown as thin lines with grey carbons. At the position of Leu139 in AnCDA, ClCDA also has a leucine (shown), while SpPgdA has a glycine and VcCDA has a threonine (both not shown). At the position indicated by Ala159 in AnCDA, ClCDA has a threonine whereas the other two CDAs have a tryptophan (all shown). At the position indicated by Leu194 in AnCDA, the other three CDAs also have leucine (all shown). At the position indicated by Lys164 in AnCDA, ClCDA also has a lysine, whereas SpPgdA and VcCDA have leucine (shown) and alanine (not shown), respectively. (d) Surface representation of AnCDA, showing the active site residues (H97, H101, D48, D47, H196) in blue with subsite numbering. The yellow area corresponds to the hydrophobic patch (Y53, Y200, W201) discussed in the text.
