Figure 4.
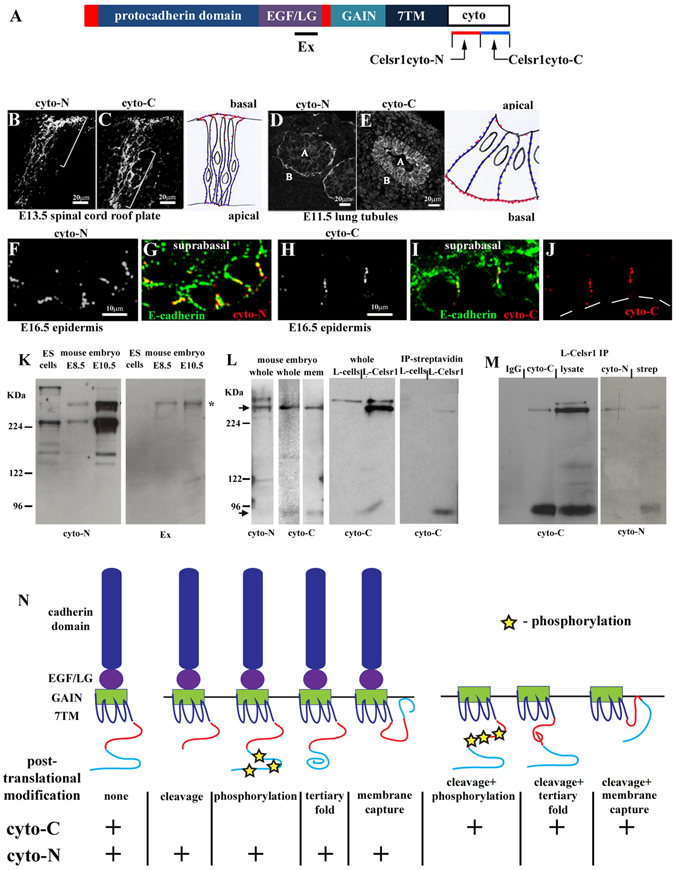
Cellular and molecular evidence for post-translational variants of Celsr1 protein. (A) Schematic of Celsr1 protein. Cysteine-rich domains (red), EGF; epidermal growth factor-like domain, LG; laminin-G like domain, GAIN; GPCR auto-proteolytic inducing domain, 7TM; seven-pass transmembrane domain, cyto tail; cytoplasmic tail. Regions of the cytoplasmic tail against which Celsr1-cyto-N and Celsr1-cyto-C antibodies were raised are marked by red and blue bars respectively. A polyclonal antibody against an extracellular LG domain is also shown. (B–I) immunostaining of frozen sections from different mouse tissues, (B–E) transverse sections (F–I) longitudinal sections. (B,C) representative images, n > 3. Consecutive frozen sections of the developing spinal cord were used to compare cyto-N and cyto-C staining patterns. Brackets label zones of cyto-N and cyto-C enrichment within the roof plate of the embryonic spinal cord. Boxed area outlines roof plate region, dorsal is to the top. Schematic to right of (B,C) highlights zones of cyto-N (red) and cyto-C (blue) enrichment in roof-plate neuroepithelial cells. (D,E) representative images, n > 3. A labels apical domain of tubule, B labels basal domain of tubule. Schematic to right of (D,E) highlights zones of cyto-N (red) and cyto-C (blue) enrichment in lung tubule cells. (F–J) representative images, n > 3. White lines denote basal lamina underlying Celsr1-expressing basal progenitor cells (basal monolayer). (K) the same tissue extracts were used for Western blot analysis of cyto-N and Ex antibodies. Asterisk labels Celsr1 p400 protein, n = 3. (L) L-Celsr1 denotes stable Celsr1 expressing L cells, IP is immunoprecipitation, n = 3 for each set of blots. Arrows mark Celsr1 p400 and p85 protein species. (M) Negative control was IgG. Specific binding of Celsr1 antibodies to Western blots was visualised using Protein A-HRP, n = 3 for each set of blots. Notably, cyto-N recognised denatured p85 when cell surface-tagged proteins were enriched from the same batch of Celsr1-expressing cells as immunoprecipitation reactions. (N) schematic showing predicted post-translational modifications of Celsr1 protein which we believe may underpin the differential recognition of native Celsr1 protein by cyto-C and cyto-N antibodies. Both antibodies could recognise a non-modified protein. Post-translational modifications could remove or block antibody-specific epitopes of the native Celsr1 protein. Thus cyto-C may not favour some large Celsr1 protein species because of C-terminal cleavage, site-specific phosphorylation, a change in tertiary protein folding or membrane capture of the C-terminal cytoplasmic tail. Equally, similar mechanisms linked to the N-terminal cytoplasmic tail of Celsr1 could prevent cyto-N-binding to p85 in its native form following protein cleavage. Membrane capture of the cytoplasmic tail of 7TM proteins can occur via post-translational modification of C-terminal tail cysteines with palmitic acid. Such palmitoylation could create a new intracellular loop48 to regulate 7TM protein endocytosis and trafficking49. Annotated Celsr1 tail sequence is shown in Supplementary Fig. S2H.
